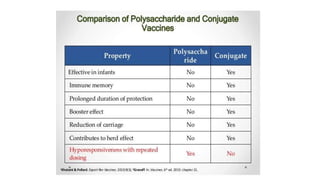
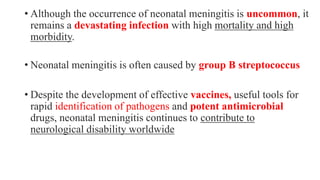
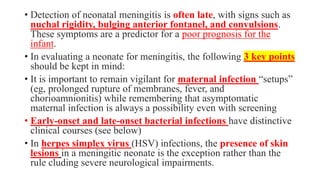
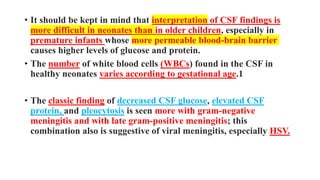
This document discusses neonatal meningitis, including its causes, presentation, diagnosis, and treatment. Group B streptococcus and E. coli are common causes. Signs can be subtle but include fever, irritability, and hypotension. Diagnosis involves lumbar puncture to examine CSF, where pleocytosis and low glucose indicate infection. Complications include cerebral edema, hydrocephalus, and neurological impairments. Treatment requires early, aggressive antimicrobial therapy for at least 2 weeks. Long-term monitoring is also needed due to potential cognitive and developmental issues.



![Etiology
Common bacterial pathogens
• group B streptococci (GBS) are the most commonly
identified causes of bacterial meningitis, implicated in
roughly 50% of all cases[5] . Escherichia coli accounts for
another 20%[6].
• Listeria monocytogenes is the third most common pathogen,
accounting for 5–10% of cases; [6].
• Studies suggest that in underdeveloped countries, gram-
negative bacilli—specifically, Klebsiella organisms and E
coli —may be more common than GBS[7]](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-5-320.jpg)
![Epidemiology
• The incidence of neonatal meningitis is estimated to be 0.3
per 1000 live births, as observed in the United States,
Sweden, The Netherlands, England, and Wales. [4
• Reported estimated incidences of neonatal meningitis that
ranged from 0.48 per 1000 live births in Hong Kong to 2.4
per 1000 live births in Kuwait. [7]
•](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-6-320.jpg)




![Bacterial meningitis
Early onset
• in the first 48 hours of life
• temperature instability, episodes of apnea or bradycardia,
hypotension, feeding difficulty, hepatic dysfunction, and
irritability alternating with lethargy. [1]
• Respiratory symptoms can become prominent within hours of
birth in group B streptococcal (GBS) infection; however, the
symptom complex also is seen with infection by E
coli or Listeria species.](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-13-320.jpg)

![• HSV meningitis
• Early features of HSV meningitis may mimic with bacterial
meningitis, including
• pallor, irritability, high-pitched cry, respiratory distress,
• fever, or jaundice, progressing to pneumonitis,
• seizures, hepatic dysfunction,
• and disseminated intravascular coagulopathy
• (DIC). [16]](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-15-320.jpg)
![Complications
• cerebral edema, hydrocephalus, hemorrhage, ventriculitis (especially with
bacterial infection), abscess formation, and cerebral infarction.
• Cerebral edema, hydrocephalus, and hemorrhage each may cause increased intracranial
pressure, with potential for secondary ischemic injury to the brain because of decreased
brain perfusion:
• Cerebral edema results from vasogenic changes, cytotoxic cell injury, and, at times,
inappropriate antidiuretic hormone (ADH) secretion
• Hydrocephalus results from debris obstructing the flow of cerebrospinal fluid (CSF)
through the ventricular system or from dysfunction of arachnoid villi; it occurs in as many
as 24% of neonates with bacterial meningitis [22]
• Hemorrhage occurs in regions of infarction or necrosis and should be suspected in a
neonate with new focal neurological findings or clinical deterioration](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-16-320.jpg)
![• Ventriculitis results in sequestration of infection to areas that are poorly
accessible to systemic antimicrobial drugs.
• Inflammation of the ependymal lining of ventricles often obstructs CSF flow.
Thus, all of these complications are interactive, making effective management
difficult.
• Ventriculitis occurs in as many as 20% of infected neonates. [23]
• Failure to respond to appropriate antibiotic therapy and signs of elevated
intracranial pressure (ICP) may suggest the diagnosis. [24]
• Intraventricular administration of antibiotics may be necessary in cases of
ventriculitis.](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-17-320.jpg)
![• Cerebral abscess occurs in as many as 13% of neonates with
meningitis. [22]
• New seizures, signs of elevated ICP, or new focal neurological signs suggest
the diagnosis.
• Brain imaging with contrast is essential for making the definitive diagnosis.
Surgical intervention may be required.](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-18-320.jpg)
![• Cerebral abscess occurs in as many as 13% of neonates with
meningitis. [22]
• New seizures, signs of elevated ICP, or new focal neurological signs suggest
the diagnosis.
• Brain imaging with contrast is essential for making the definitive diagnosis.
Surgical intervention may be required.](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-19-320.jpg)
![• Cerebral infarction, both focal (arterial) and diffuse (venous), may
complicate recovery.
• Autopsy studies have found evidence of infarction in 30-50% of specimens
studied. [1]
• Imaging studies suggest that the actual incidence of infarction may be even
higher. [25]
• Meningitis has been shown to be associated with 1.6% of all cases of neonatal
arterial stroke and 7.7% of venous infarcts. [26]
• Necrotizing lesions secondary to HSV meningitis can be deleterious to the
developing brain.](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-20-320.jpg)
![• longer-term complications that may develop include residual epilepsy,
cognitive impairment, hearing loss, visual impairment, spastic
paresis, and microcephaly. Some of these disorders may be difficult to
detect during infancy.
• Hearing, for example, is difficult to evaluate without the child’s
cooperation, and even then, assessment may be limited to behavioral
response to sounds.
• Brainstem auditory evoked response (BAER) testing does not evaluate
all dimensions of hearing, but this test, which can be performed reliably
in sedated infants, only slightly overestimates hearing loss, which
occurs in 30% of survivors of bacterial meningitis and 14% of
survivors of nonbacterial meningitis. [27] Subtle impairment of sound
discrimination may not be readily apparent.](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-21-320.jpg)
![Long-Term Monitoring
• Because of the potential for hearing loss, neonates with meningitis
should undergo brainstem auditory evoked response (BAER)
testing at 4-6 weeks after discharge. [40]
• Survivors of neonatal meningitis require long-term surveillance
not only for disorders of hearing but also for disorders of vision,
motor, or cognitive function.
• Developmental delay is a frequent complication of neonatal
meningitis.
• Early intervention services should be employed to maximize
developmental gains](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-22-320.jpg)
![• cognitive impairment may not be evident until the child has started school
or advanced into higher grades where more complex analysis of information is
necessary. [20]
• Careful screening for neurological, cognitive, and developmental deficits
must be conducted as part of routine pediatric care over a period of many
years,
• and the responsible physician should be attentive to possible
problems with perception, learning, or behavior that may result
from neonatal infection.](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-23-320.jpg)
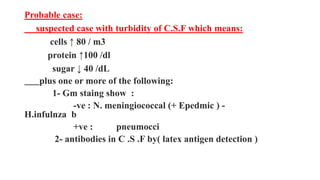
![Laboratory Studies
•Lumbar Puncture
• Lumbar puncture is indicated for evaluation of the CSF in
all neonates suspected of having sepsis or meningitis, even
in the absence of neurological signs.
• Many clinicians are reluctant to perform this procedure on
a critically ill infant. Although the theoretical complications
of lumbar puncture include trauma, brain-stem herniation,
introduction of infection, and hypoxic stress, none of these
complications were reported in a meta-analysis of more
than 10,000 infants who underwent lumbar puncture. [29]
• .](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-24-320.jpg)
![• Meningitis, however, increases the risk of death in neonates. Stoll
et al reported a mortality of 23% in babies with CSF-proven
meningitis, compared with a mortality of 9% in neonates whose
lumbar puncture results were not consistent with meningitis. [35]
• Additionally, many infants who had negative blood cultures had
positive CSF cultures, suggesting that cases of meningitis may be
missed.
• In cases of bacterial meningitis, repeat lumbar puncture should be
performed 24-48 hours after initiation of therapy to ensure
sterilization of the CSF. After a full course of therapy for PCR-
proven HSV, repeat lumbar puncture should be undertaken to
rule out incompletely treated infections](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-25-320.jpg)


![Magnetic Resonance Imaging
• Magnetic resonance imaging (MRI) is the neuroimaging modality of
choice for identifying focal areas of infection, infarction, secondary
hemorrhage, cerebral edema, hydrocephalus, or, rarely, abscess
formation.
• Sinovenous occlusions, ventriculitis, and subdural collections are best
diagnosed with MRI.
• Follow-up MRI scans are useful for following the resolution of the
infection, as well as for contributing to prognostication. If available,
magnetic resonance spectroscopy can add important information on the
metabolic function of the neonatal brain.
• Several studies have documented periventricular white matter
abnormalities on MRI in infants with neonatal meningitis. [31]
• Newer MRI technologies, including diffusion-weighted and diffusion
tensor imaging, have allowed this association to be evaluated in more
detail, and such evaluation may prove to have prognostic
implications. [32]](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-29-320.jpg)







![Prognosis
• 25-50% have significant problems with language, motor function,
hearing, vision, and cognition [18, 3] ;
•
• 5-20% have future epilepsy. [19, 20]
• Survivors are also more likely to have subtle problems, including
visual deficits, middle-ear disease, and behavioral problems. [21]
• As many as 20% of children identified as normal at 5-year follow-
up may have significant educational difficulties lasting into late
adolescence. [18]](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-38-320.jpg)







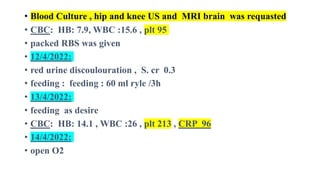

















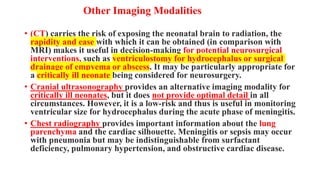
![Prevention
• The use of intrapartum antibiotic prophylaxis in pregnant mothers
who are positive for group B streptococcal (GBS) colonization on
screening or have risk factors for GBS colonization has reduced the
incidence of neonatal early-onset GBS meningitis from
approximately 1.8 cases to 0.3 cases per 1000 live births. [9]
• Screening and risk factor assessment should be included universally
in routine prenatal care.
• Cesarean delivery decreases, but does not eliminate, transmission of
HSV from the mother’s genital tract to the neonate in cases of known
infection.
• Suppressive antiviral therapy for HSV-infected women during the
third trimester may prevent recurrent infectious episodes and
thereby minimize the infant’s exposure to the virus during
delivery. [16]](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-66-320.jpg)
![Diet
• Listeria monocytogenes and Escherichia coli are bacteria found in
contaminated foods that can cause neonatal meningitis. [43]
• By avoiding certain foods and safely preparing produce, pregnant mothers
can reduce the risk of neonatal meningitis caused by these bacteria.
• Foods to avoid that may be contaminated by listeria include:
• Soft cheeses made with unpasteurized milk
• Smoked seafood that is not canned or shelf-stable
• Raw (unpasteurized) milk
• Foods that must be safely prepared to prevent the growth of listeria and E.
coli include:
• Raw sprouts
• Melons الشمام
• Hot dogs, deli meat, and cold cuts الباردة اللحوم](https://image.slidesharecdn.com/neonatalmeningitis-220830222513-ae1789e4/85/Neonatal-Meningitis-pptx-67-320.jpg)