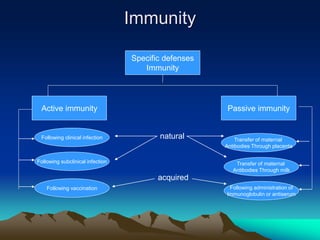
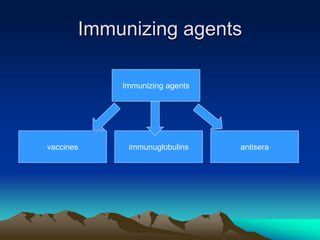
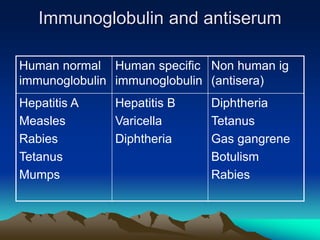
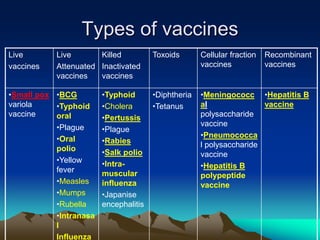
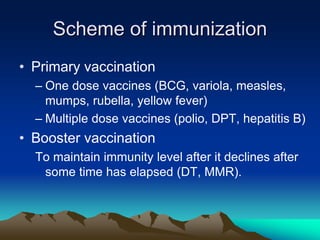
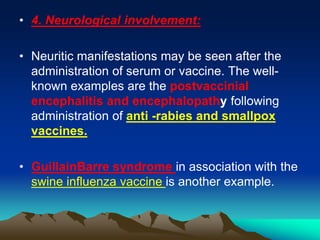
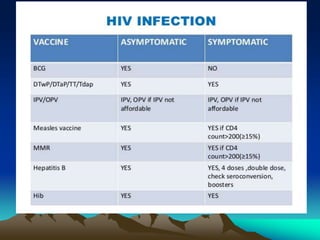
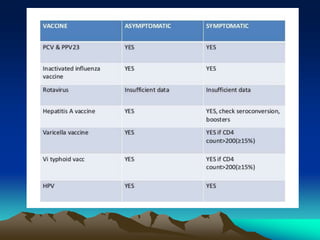
This document provides information on basic immunization and immunization in special situations. It discusses different types of immunity including active and passive immunity. It describes various immunizing agents such as immunoglobulins, antisera, and different types of vaccines including live, attenuated, inactivated, toxoids, and recombinant vaccines. It also discusses vaccination schedules, adverse reactions to immunization, maintaining the cold chain, and provides guidance on immunization for special populations such as preterm infants, immunocompromised individuals, and hepatitis B-positive mothers and their newborns.