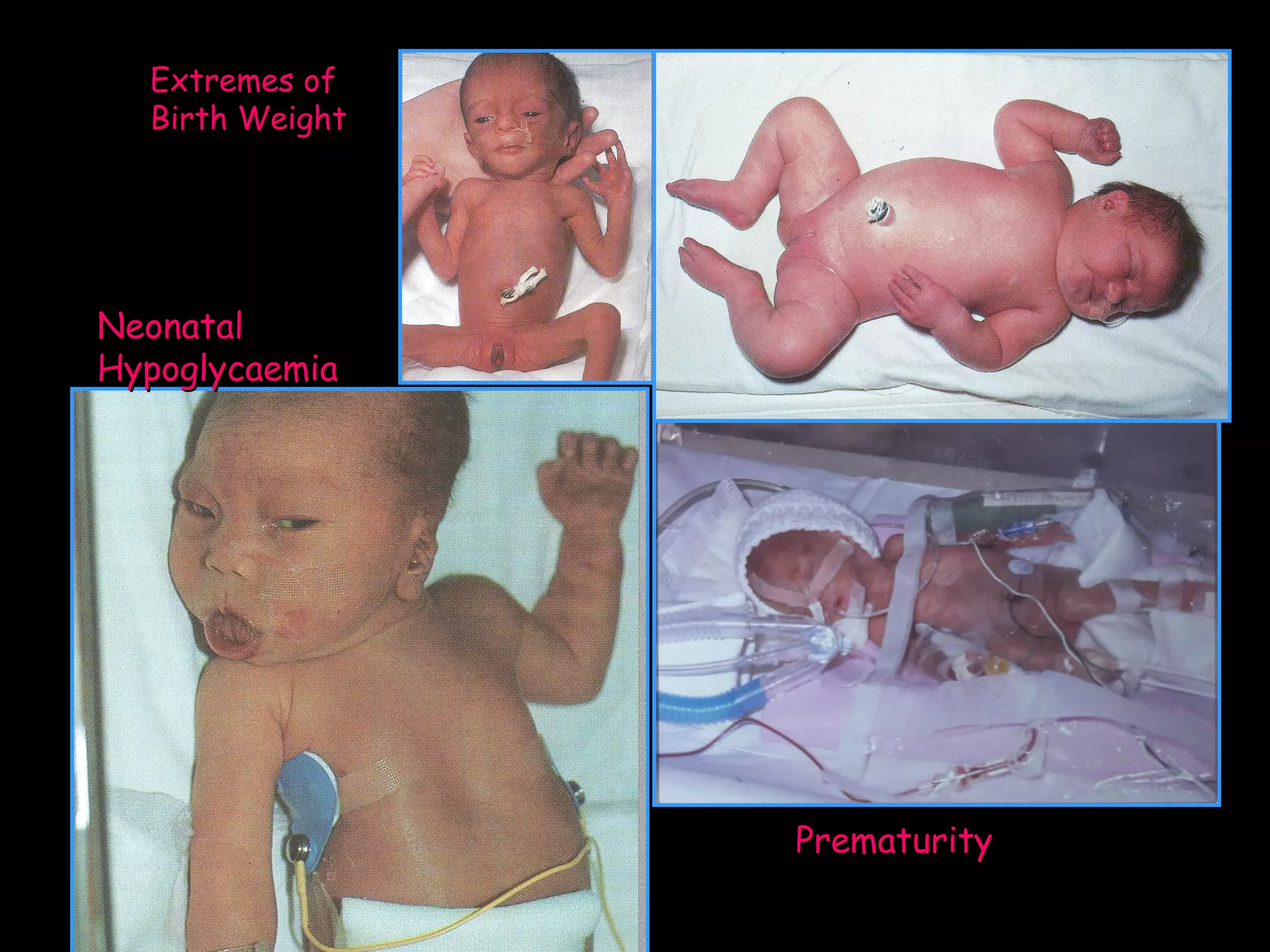
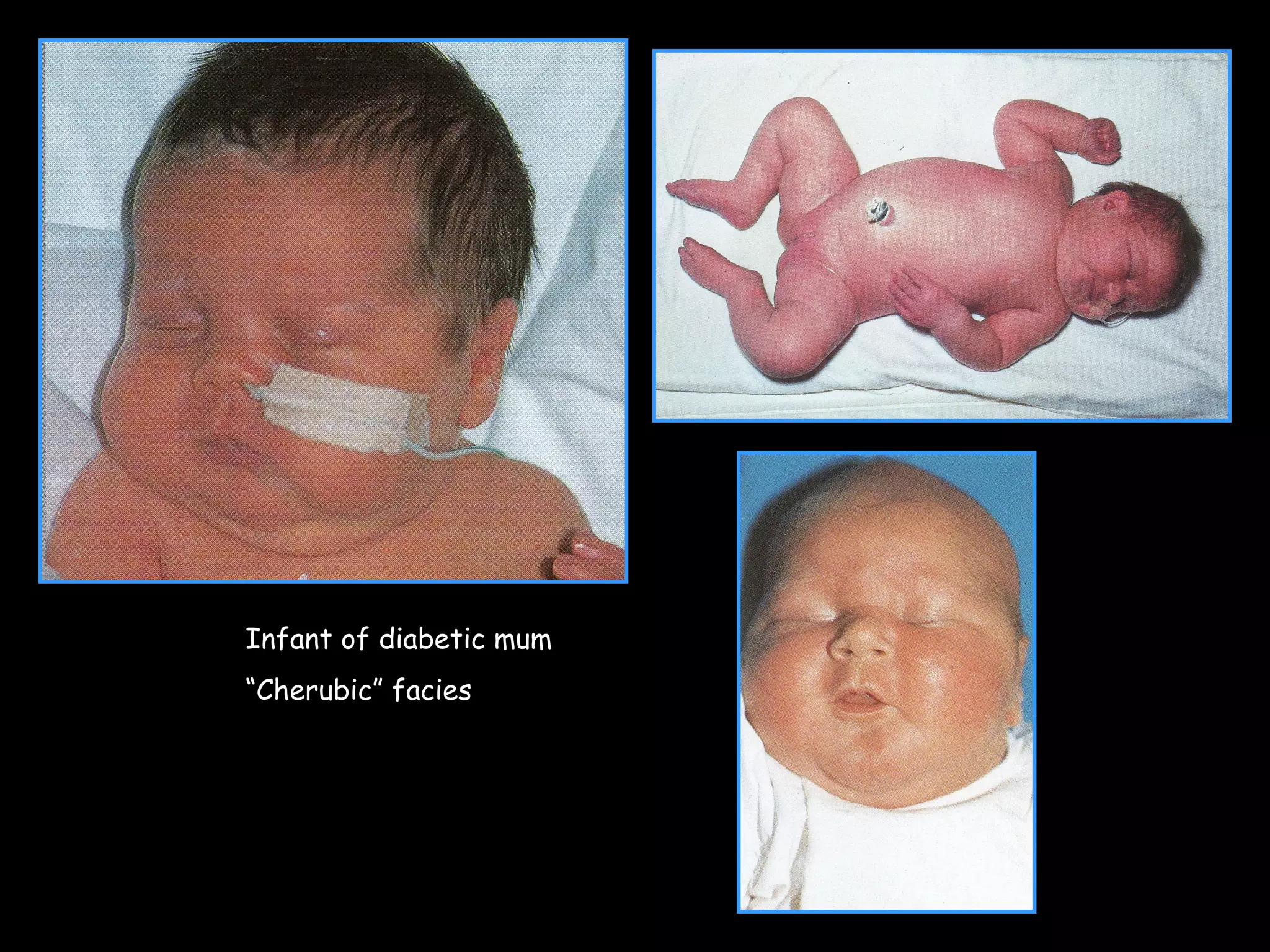
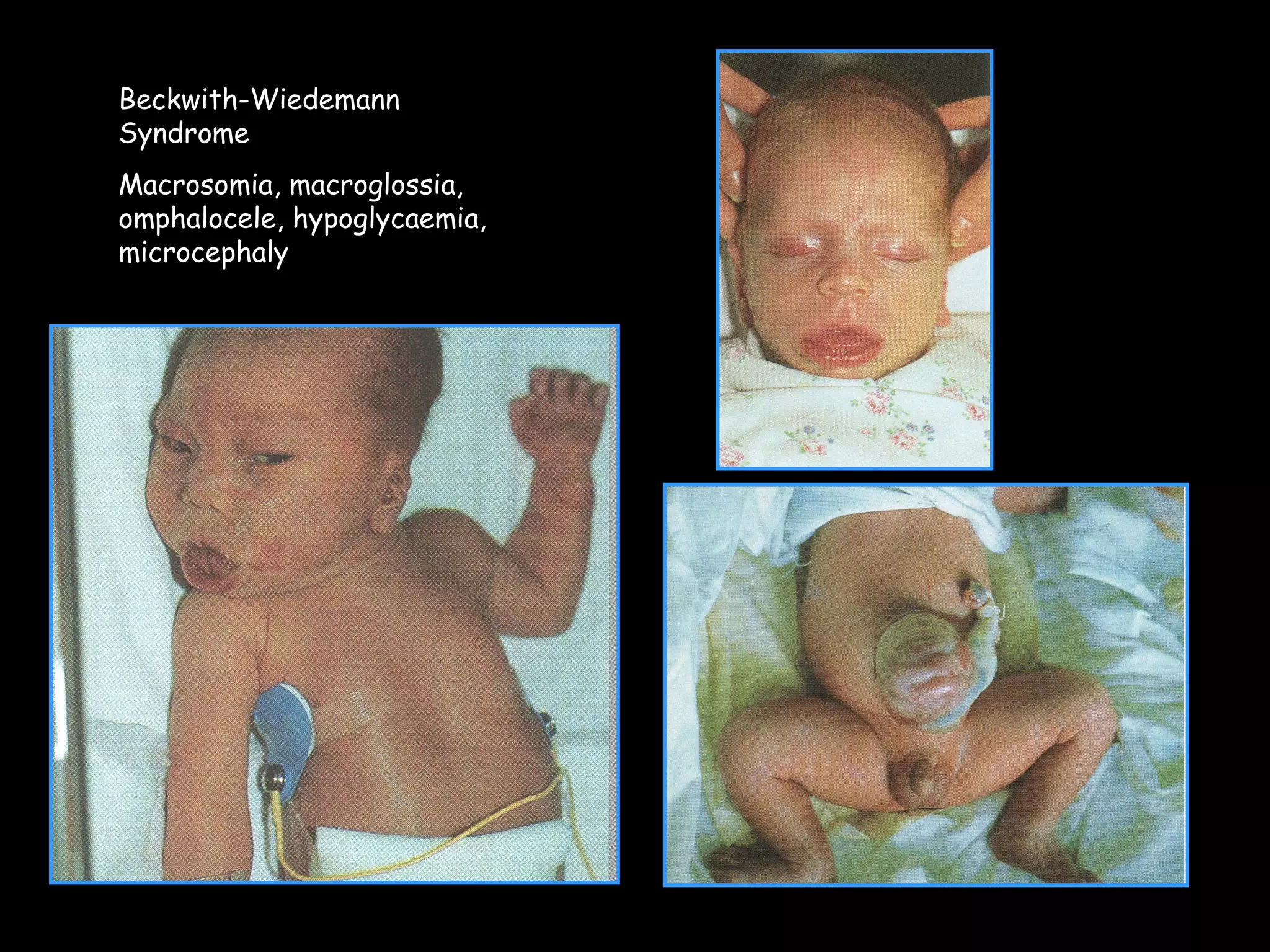
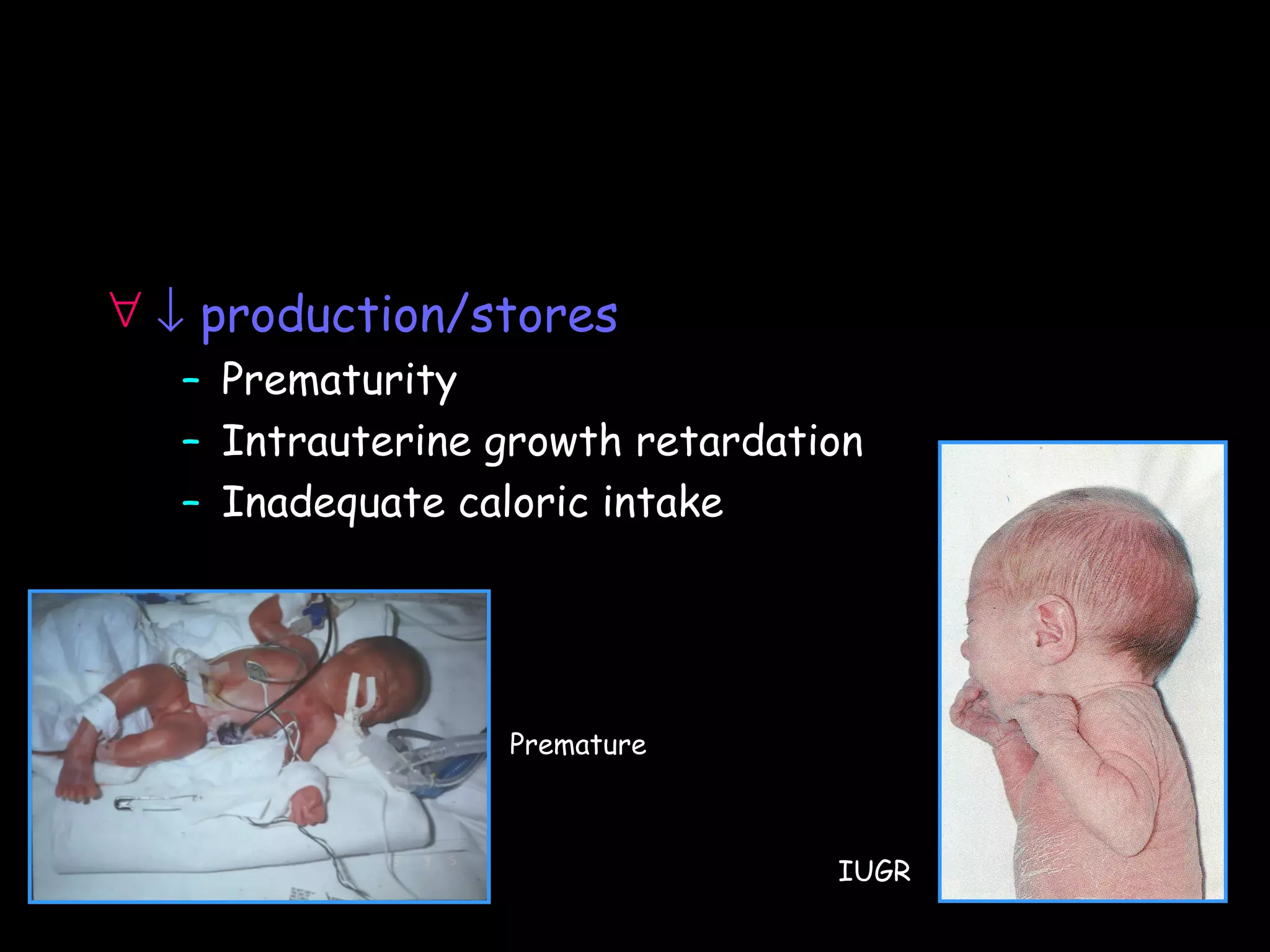
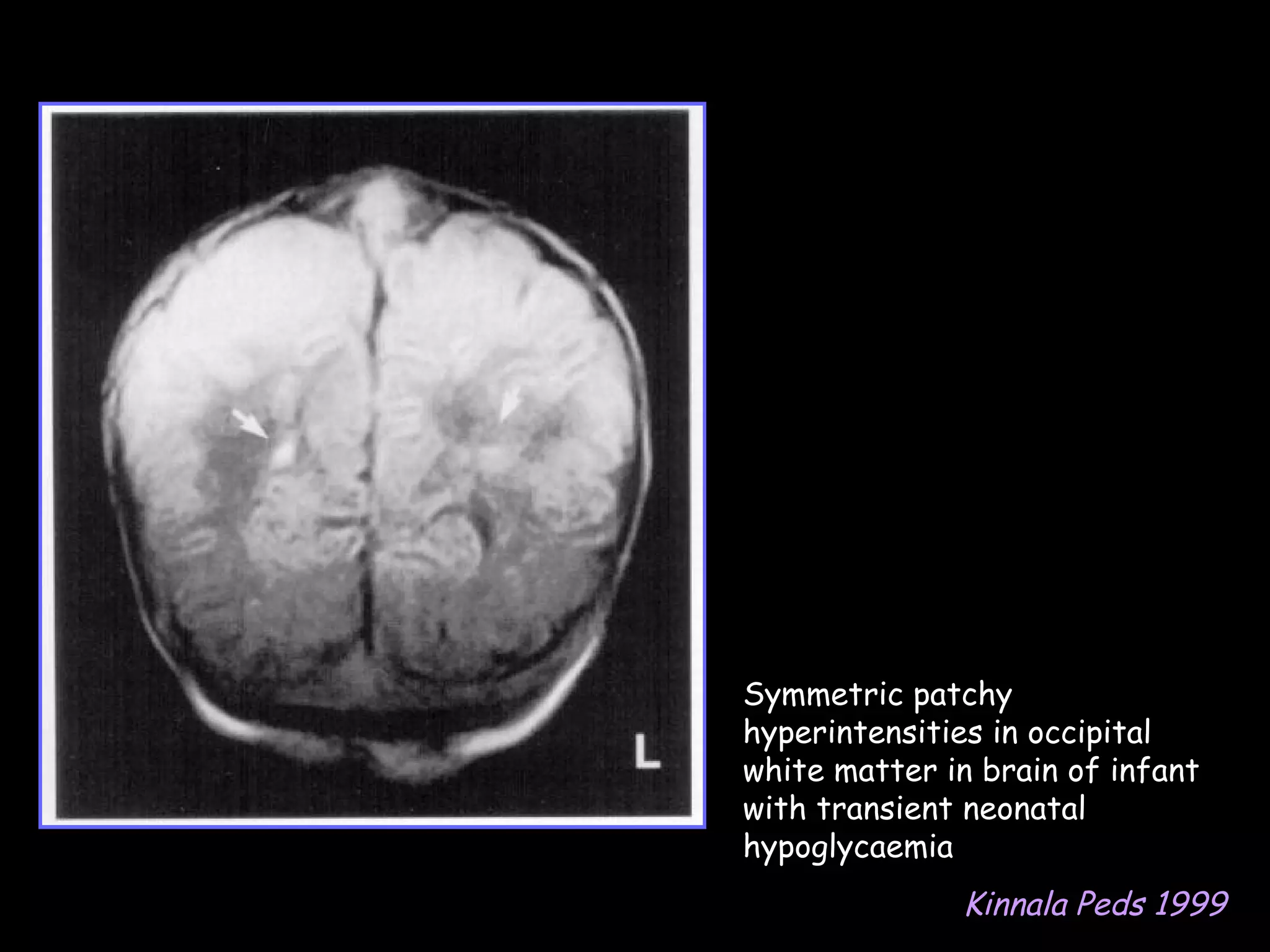
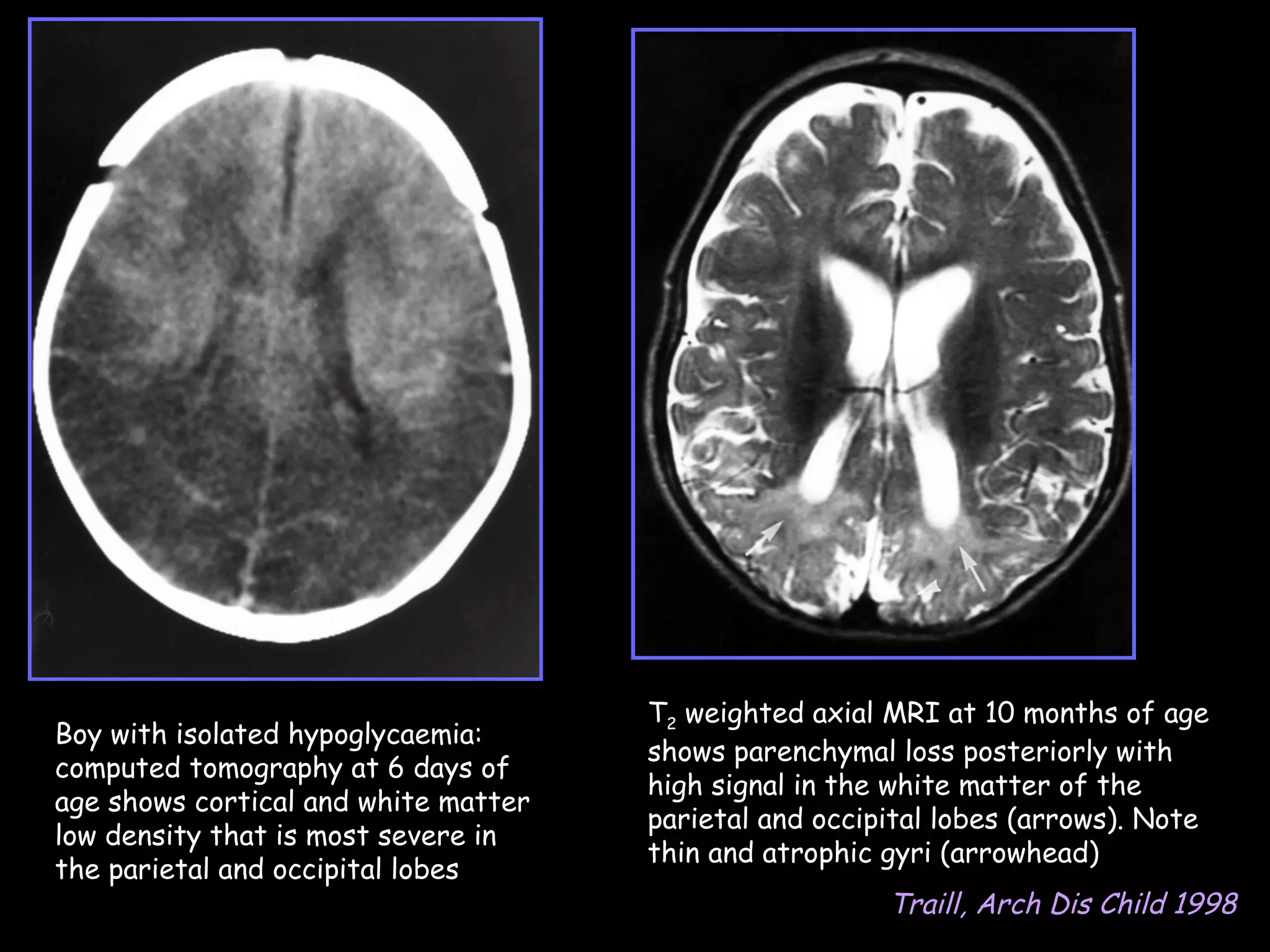
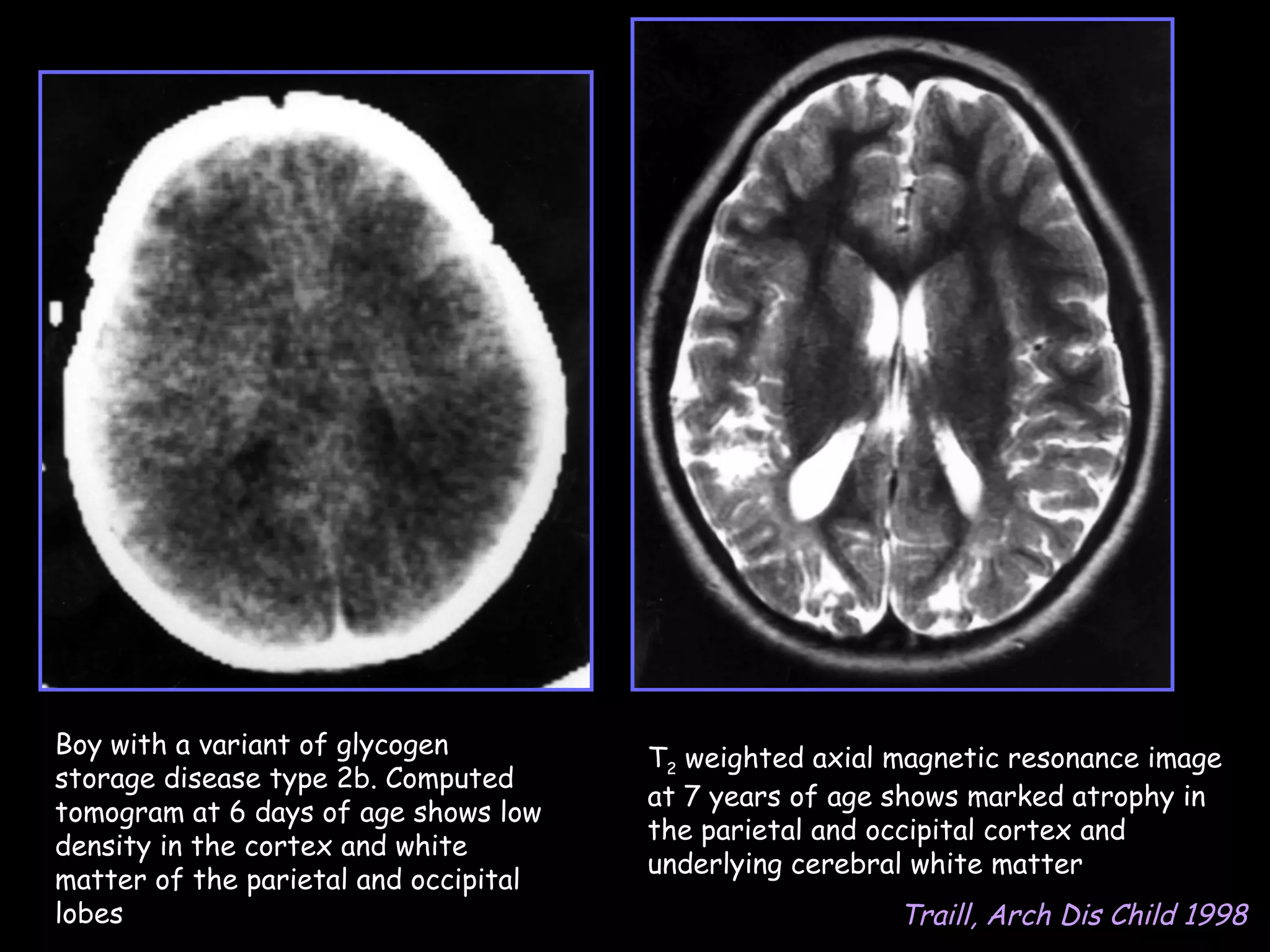
This document discusses neonatal hypoglycemia. It begins by defining neonatal hypoglycemia and noting the controversial operational thresholds used. It then describes the clinical features and various potential etiologies including increased glucose utilization, decreased production, prematurity, IUGR, and various stressors. The management sections cover prevention, anticipation through screening, diagnosis, treatment primarily with IV dextrose infusion, and adjunct therapies. It notes most cases resolve in 2-3 days but persistent or recurrent hypoglycemia may require further evaluation. The significance of hypoglycemia and potential long-term neurological outcomes are described depending on severity and duration. Long-term neurodevelopmental follow-up is recommended.