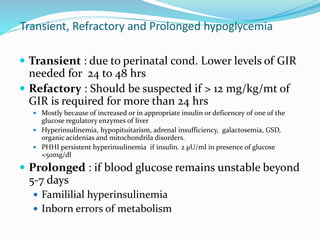
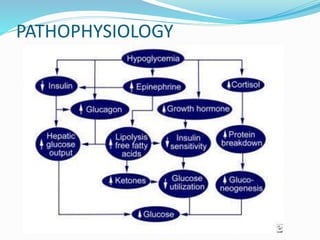
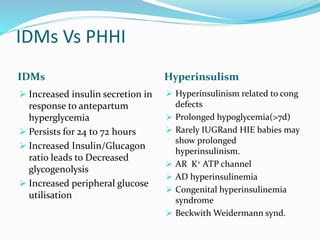
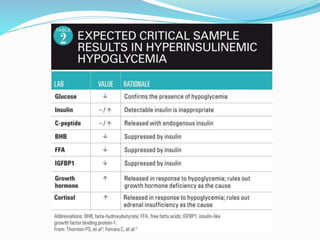
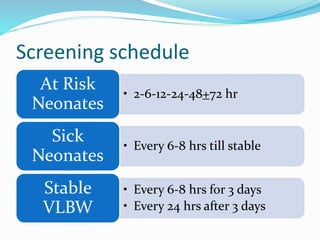
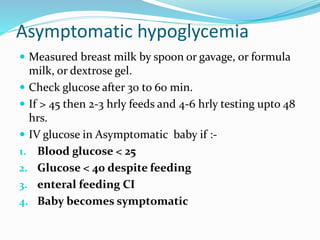
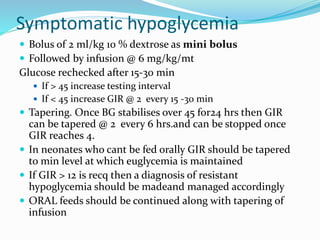
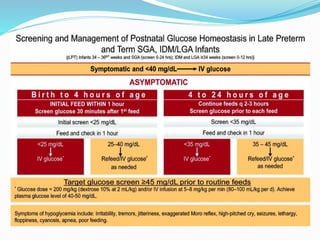
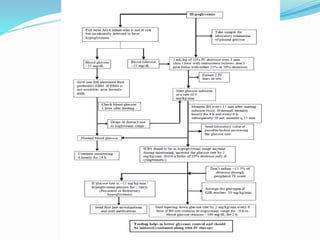
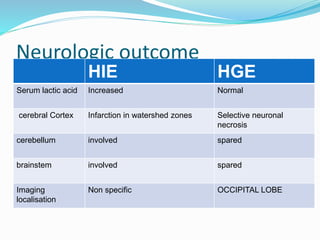
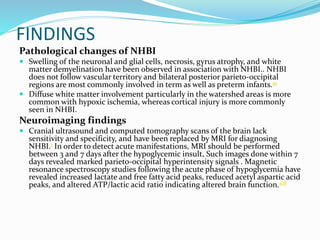
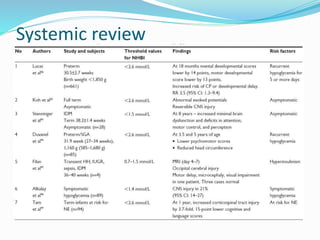
This document discusses the case of a term male infant born without complications who experienced episodes of hypoglycemia. At 2 hours of age he was jittery with a blood glucose of 35 mg/dL and improved after feeding. On the second day of life he again had low glucose of 35 mg/dL. At 2 weeks he presented with fussiness, jitteriness, staring spells, somnolence, and seizures with glucose less than 10 mg/dL, requiring IV glucose treatment. He continued having recurrent hypoglycemic episodes over the following weeks.