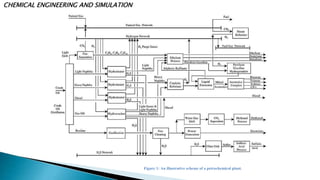
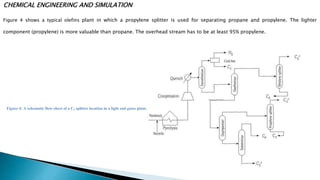
The document discusses the design and functioning of a propane-propylene splitter unit, detailing the significance of various separation techniques in chemical engineering, particularly in petrochemical processes. It emphasizes the importance of propylene as a chemical intermediate and outlines the production, purification, and uses of different grades of propylene derived from refinery processes. Additionally, it includes various operational parameters for distillation columns and methods for optimizing the separation efficiency of hydrocarbon mixtures.