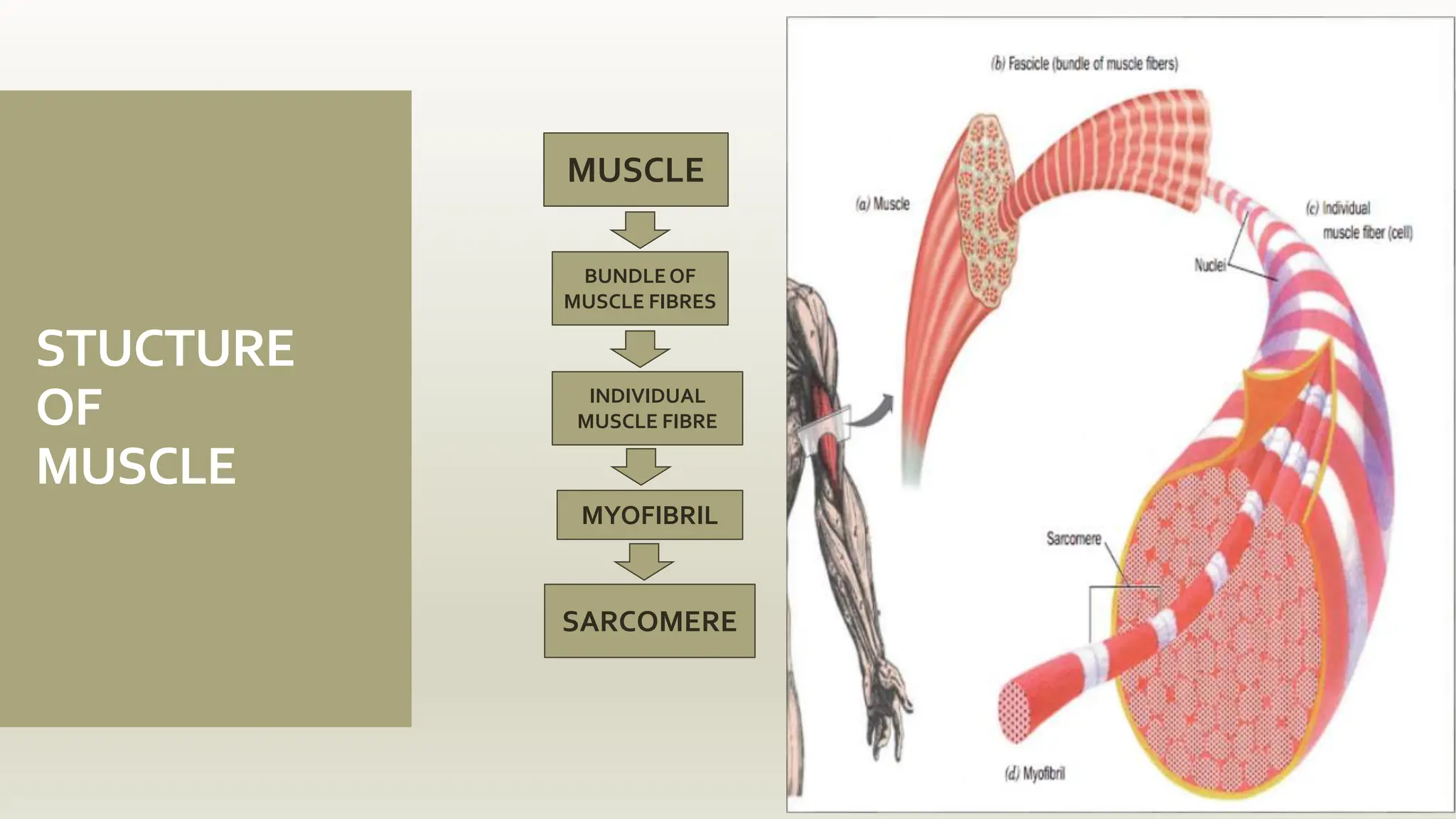
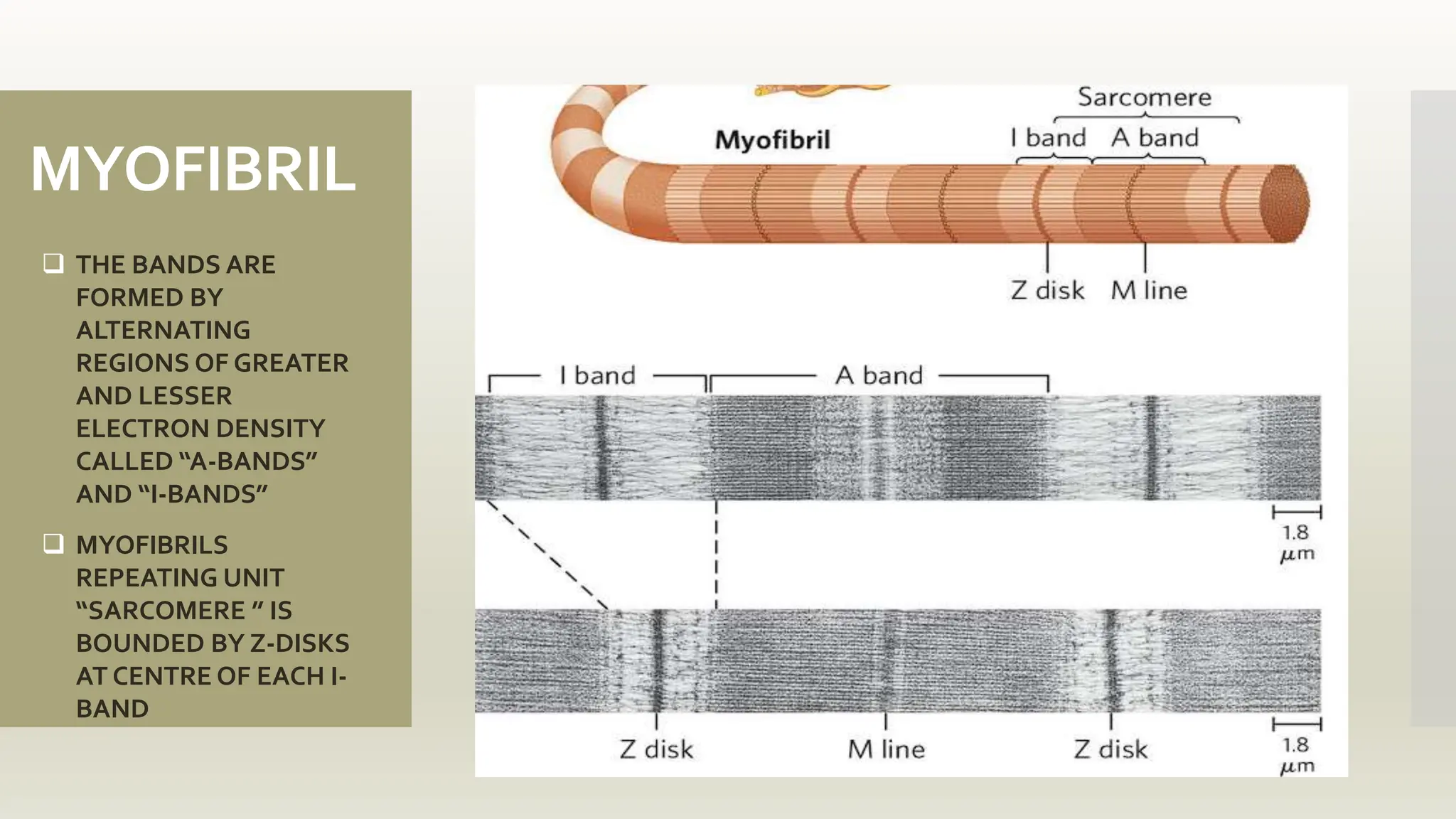
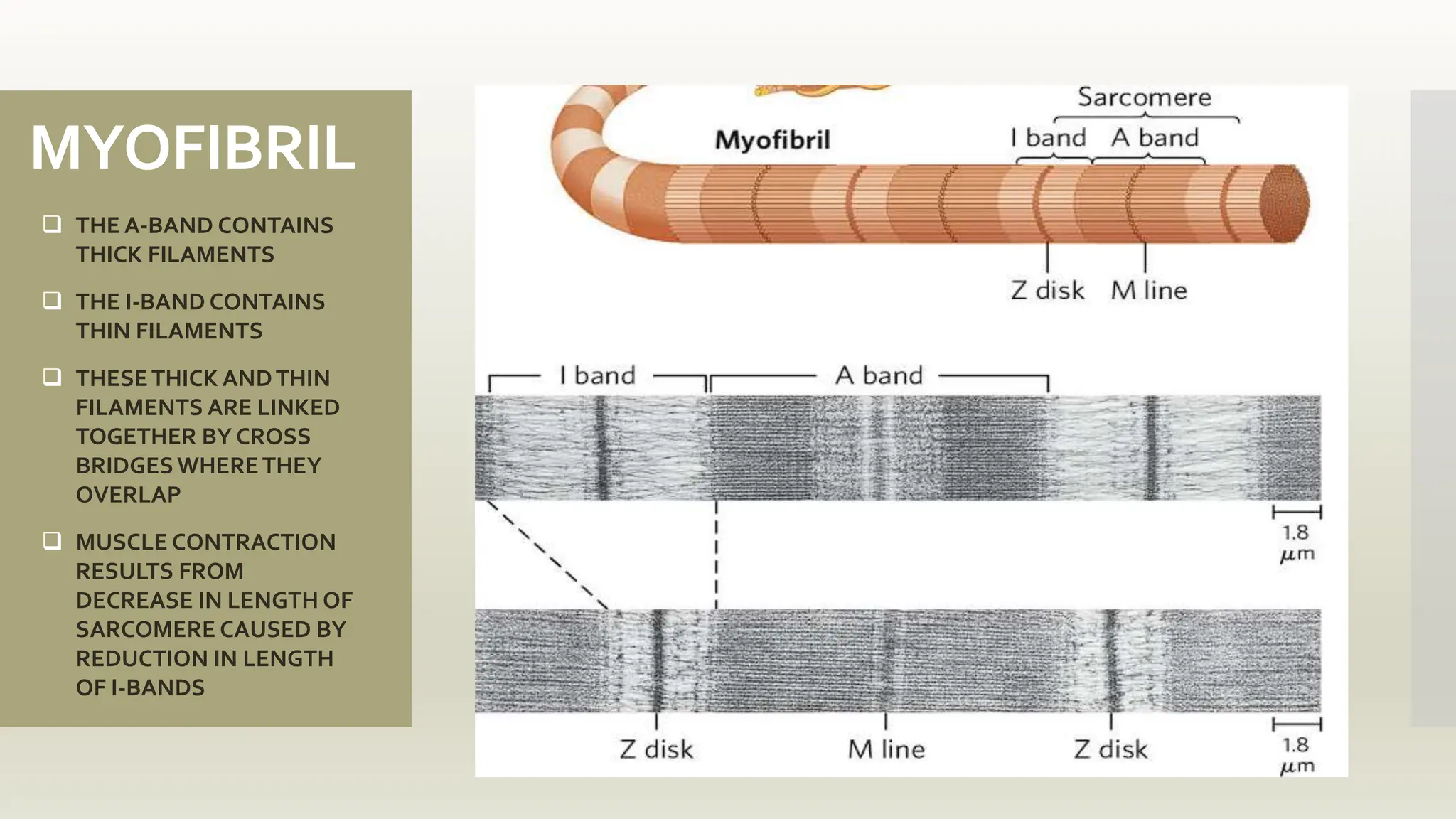
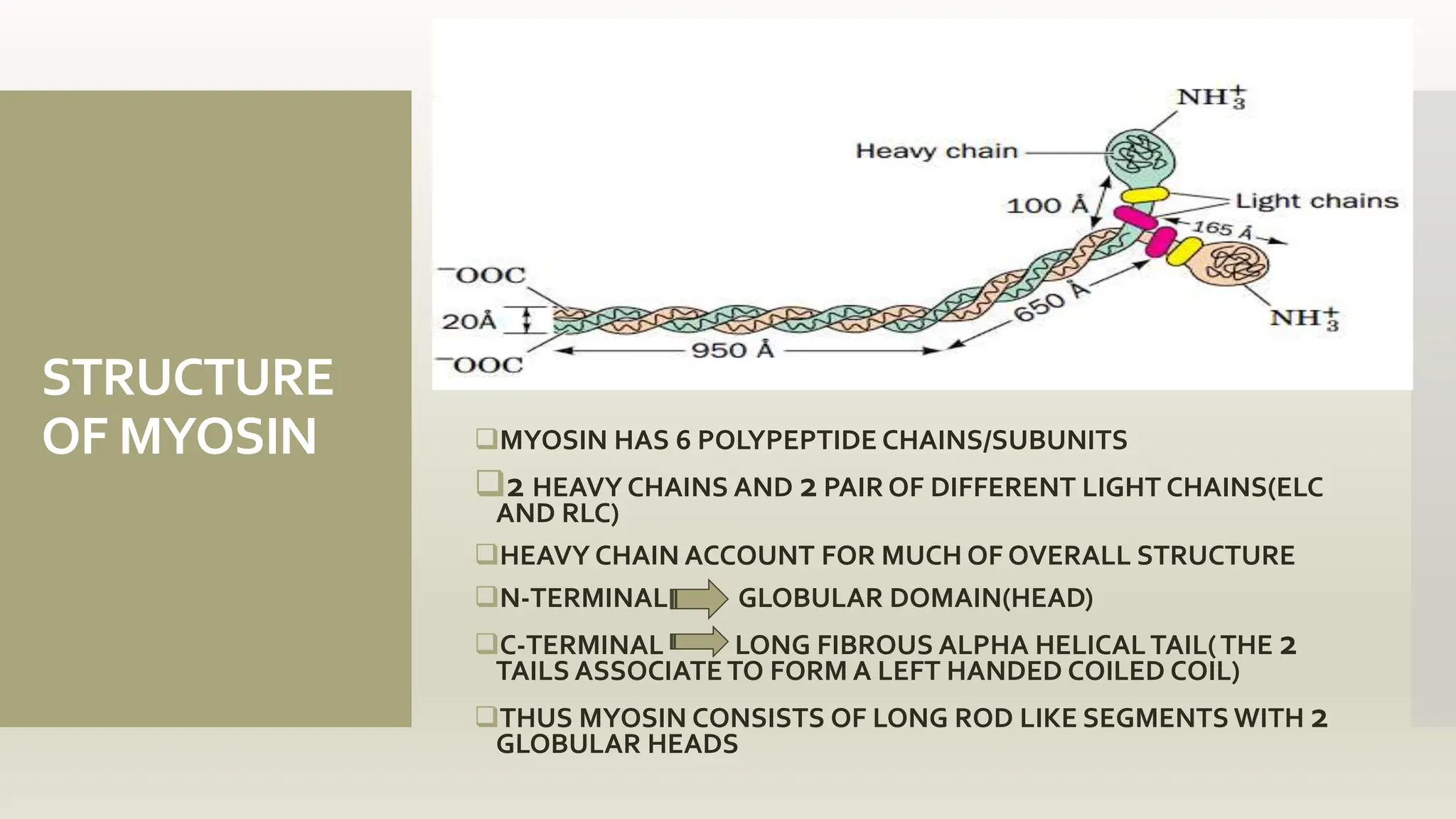
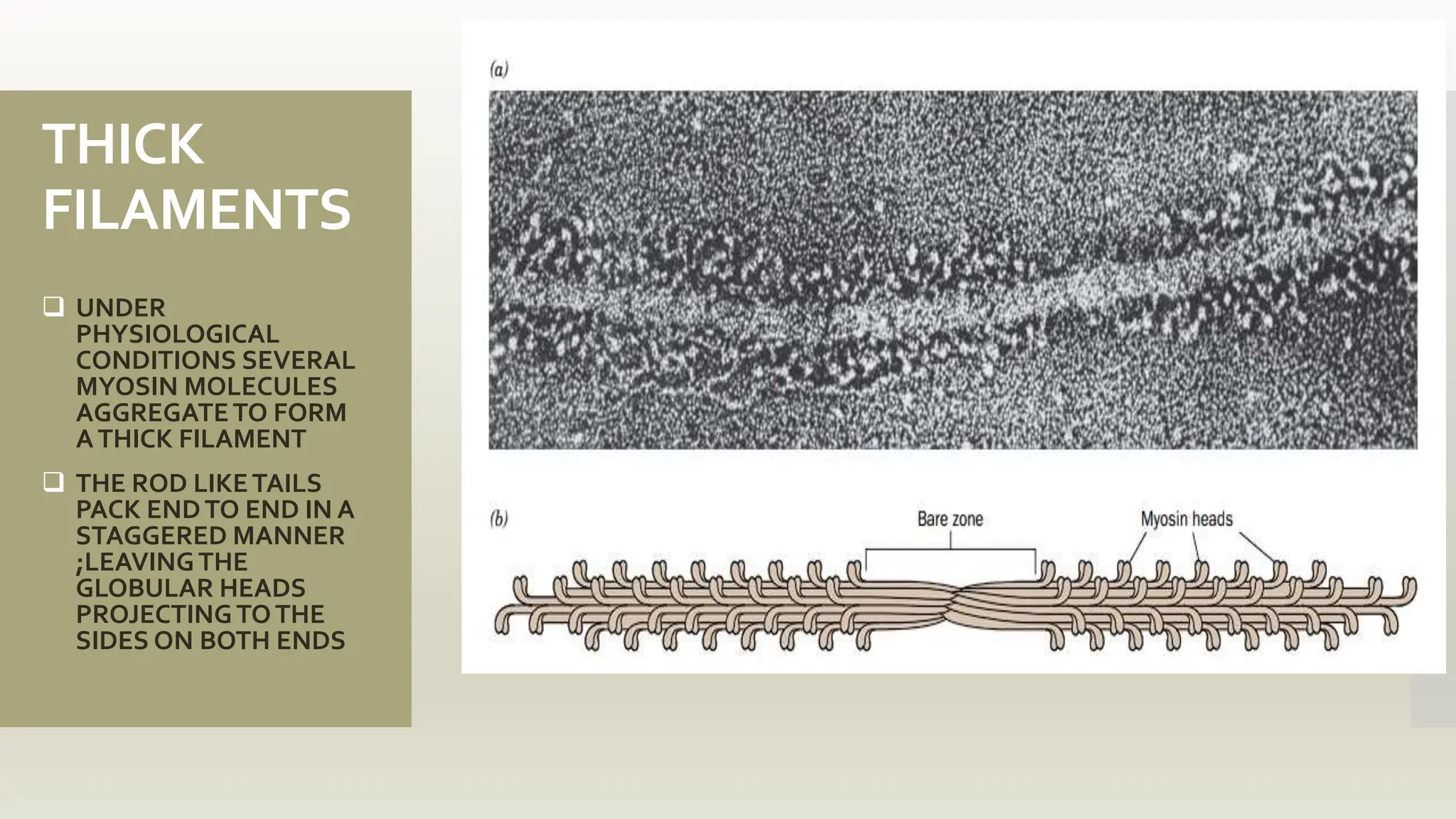
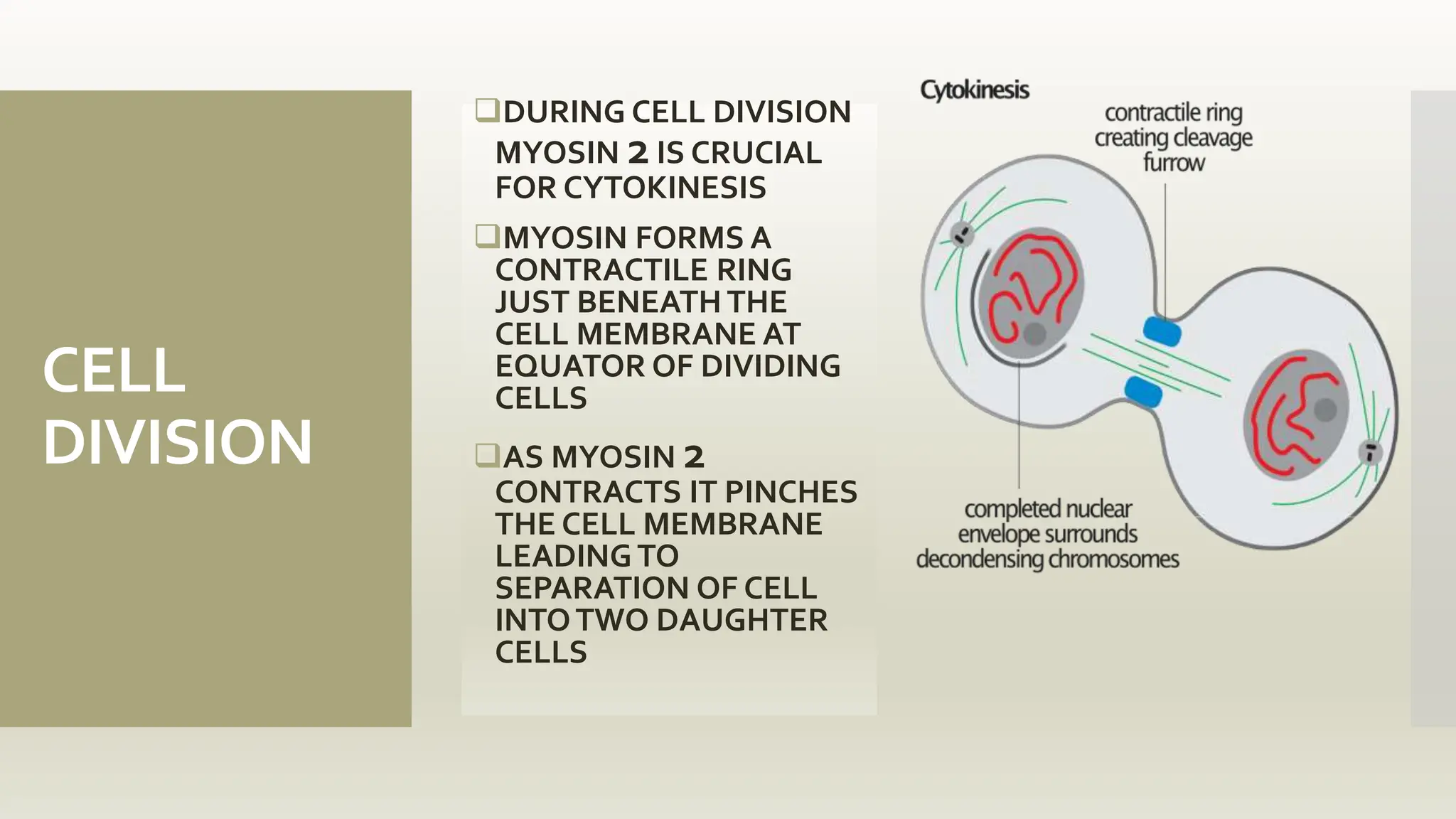
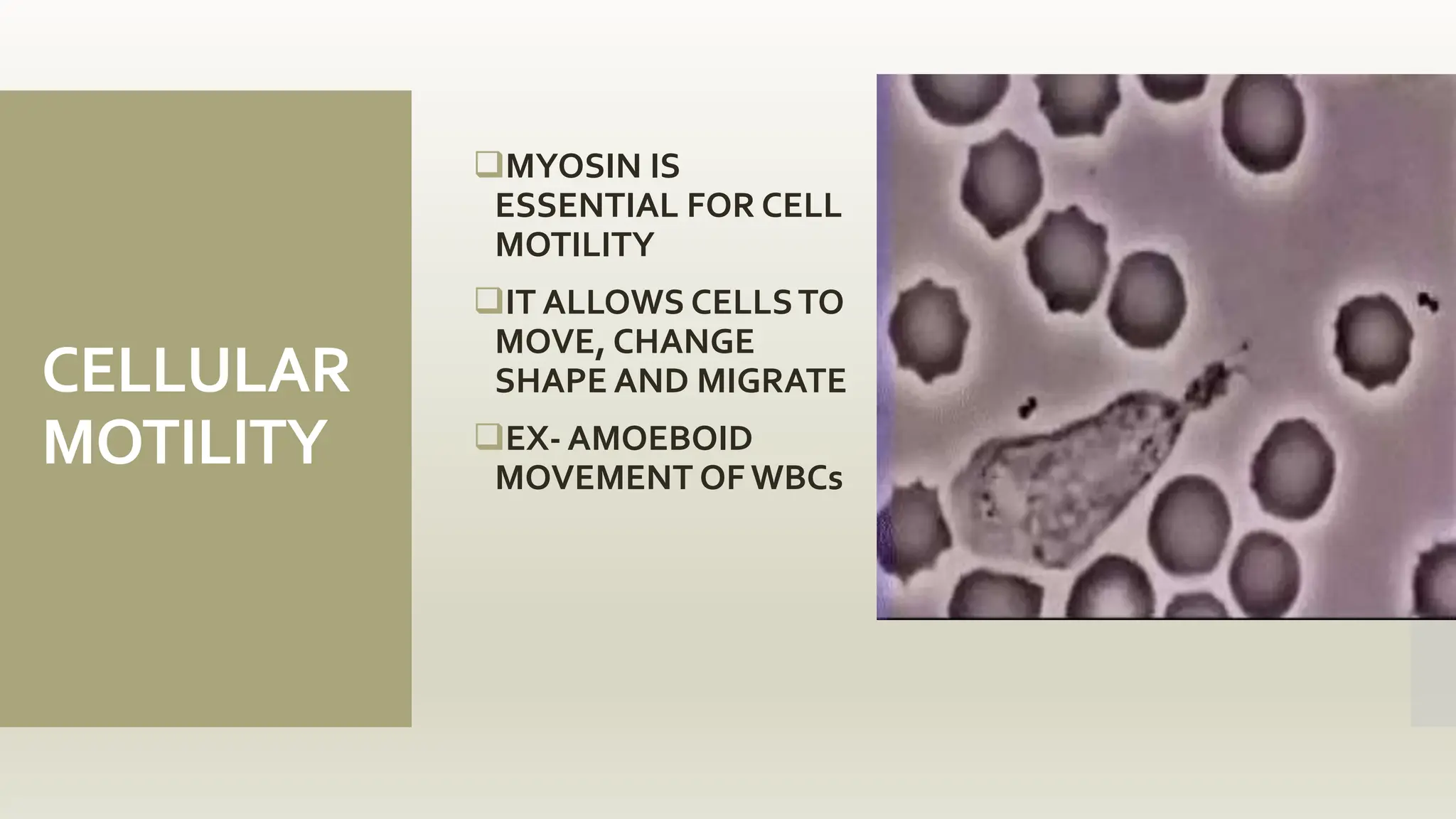
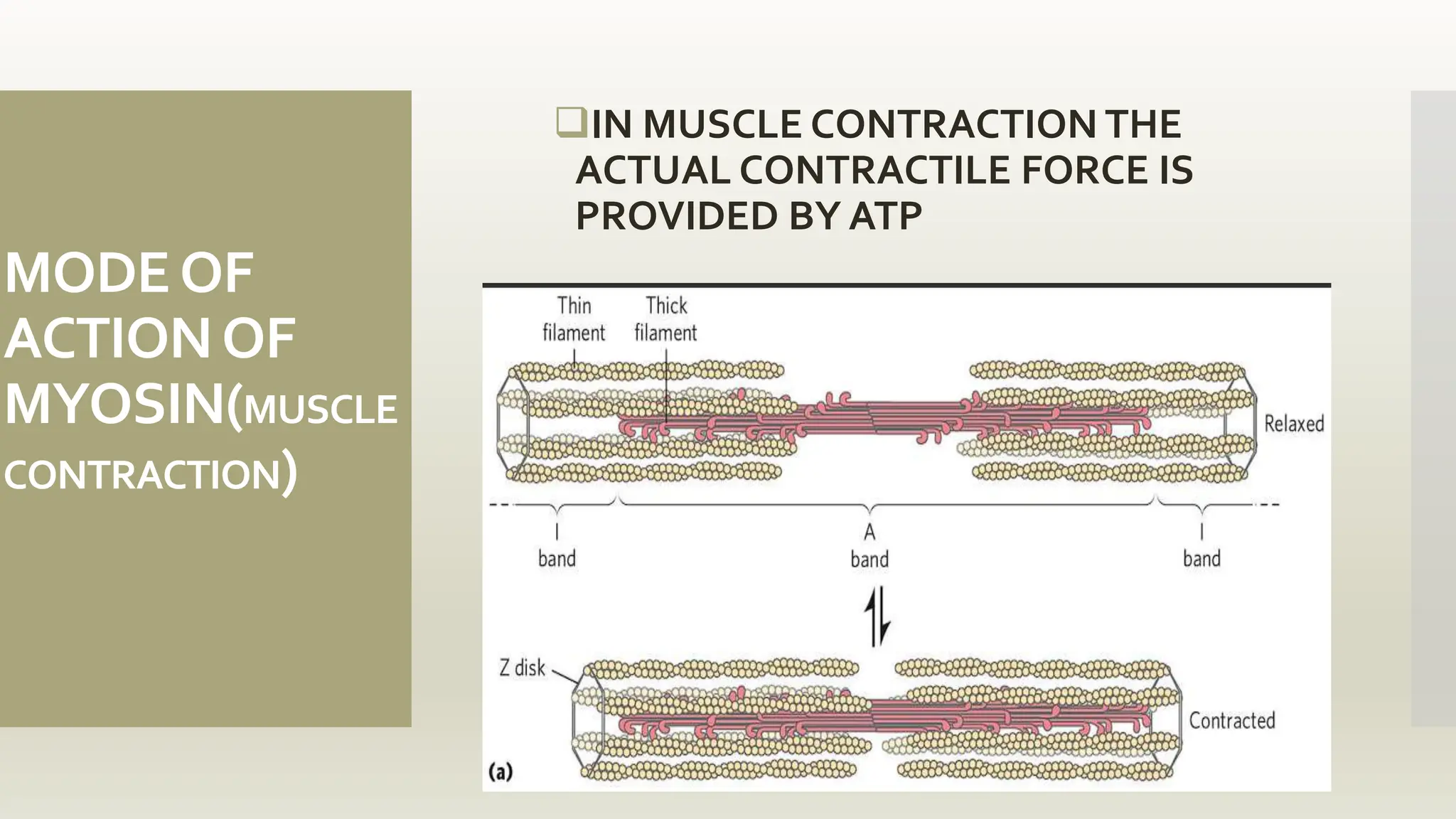
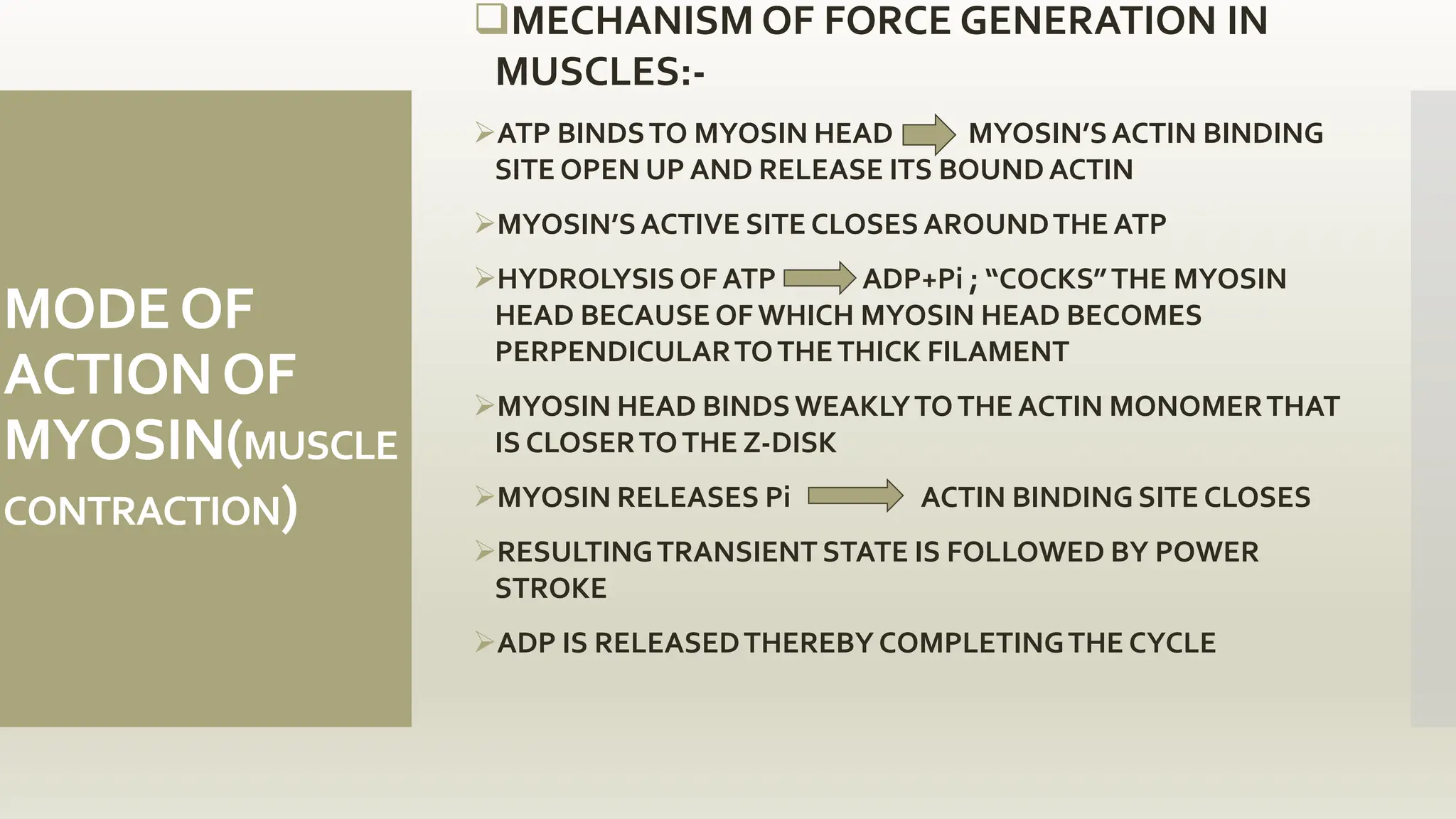
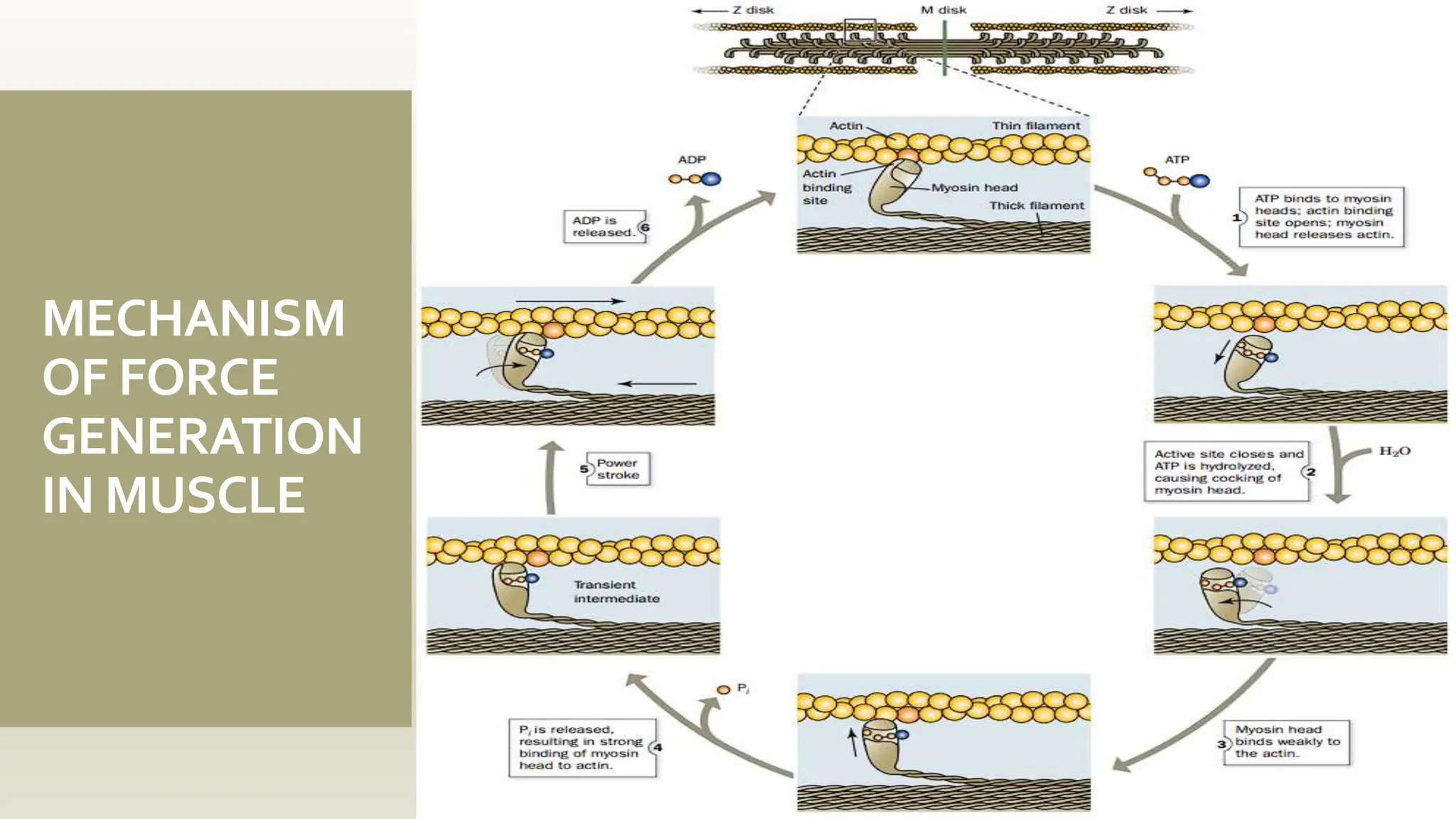
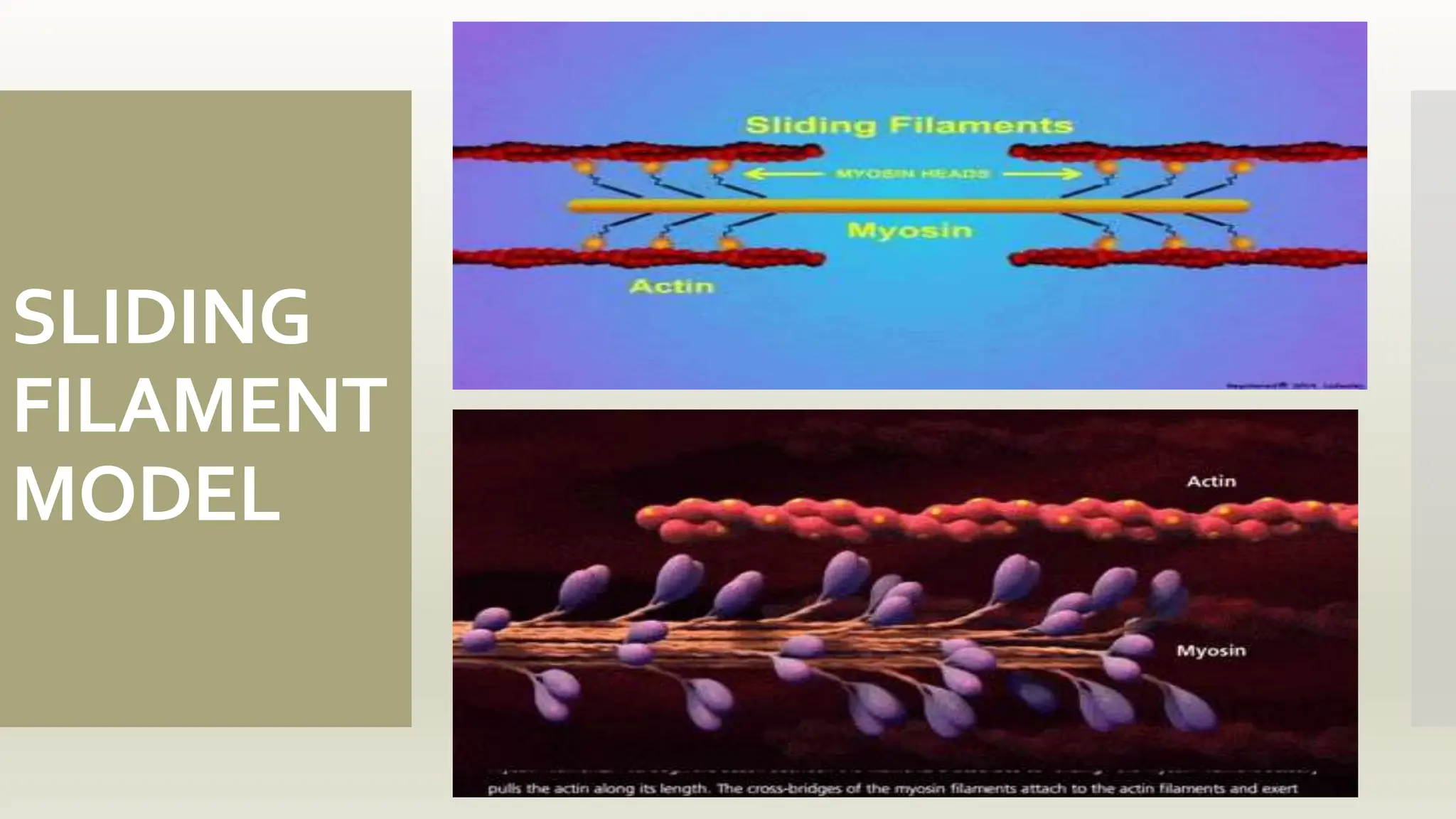
This document discusses the structure and function of myosin, a crucial motor protein in muscle contraction and various cellular processes. It details myosin's historical background, structure, and mechanisms of action, including its role in cellular transport, motility, and division. The summary also covers the sliding filament model and how ATP hydrolysis fuels muscle movement.