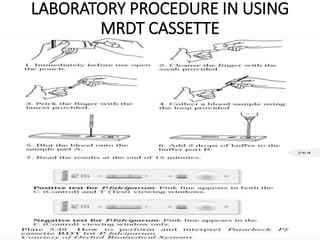
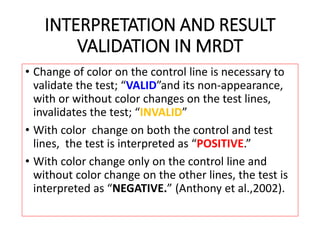
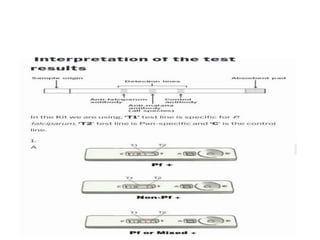
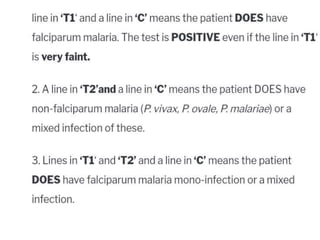
The document discusses malaria rapid diagnostic tests (MRDTs), which are immunochromatographic tests that can detect malaria antigens within 20 minutes. MRDTs use devices like cassettes or strips to detect antigens like histidine-rich protein 2, plasmodium lactate dehydrogenase, and plasmodium aldolase. They have advantages like rapid results and ease of use but also limitations such as inability to determine past vs present infections or quantify parasite density. The document outlines the components, procedure, interpretation, quality controls and appropriate uses of MRDTs to diagnose malaria.




















![LIMITATIONS OF MRDTs CONT.
The use of the three antigens results in some fundamental diagnostic limitations;
• SPECIFICITY:
• None of the 3antigens is specific for plasmodium falciparum, plasmodium ovale,
plasmodium malariae, or plasmodium knowlesi.
• GENETIC VARIATION:
• There are variants of p falciparium in south America that do not produce the 2 most
common types of HRP ( p.falciparium HRP[PfHRP-2 and HRP-3]), which means that
MRDTs based on detection of those antigens would not be useful in that region
(Gamboa et al., 2010).
• CROSS-REACTIONS
• Cross-reaction with a pfHRP-2 assay have been reported from patients with
Schistomiasis mekongi infection with no cross-reaction with pLDH assay (Leshem et
al., 2011).
• Cross-reaction with some assay has been reported with pLDH assay have been
reported for patients with rheumatoid factor or other circulating auto-antibodies
(Leshem et al., 2011).
• FALSE POSITIVITY
• Patients with high levels of P falciparium parasitaemia may give false- positive result
with PLDH assay designed to detect P. vivax (Jacobs J, 2010)](https://image.slidesharecdn.com/seminarpresentationcorrected-230614165918-3821ec38/85/MRTD-29-320.jpg)