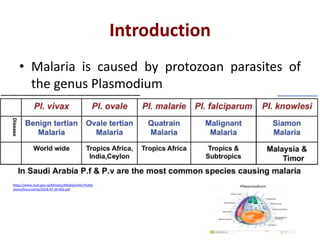
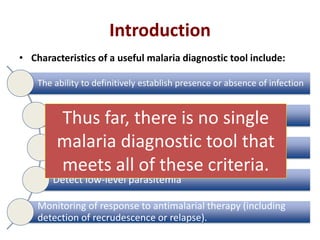
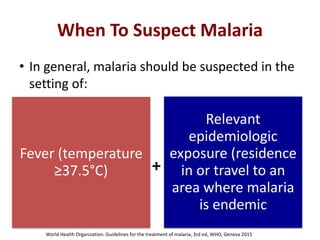
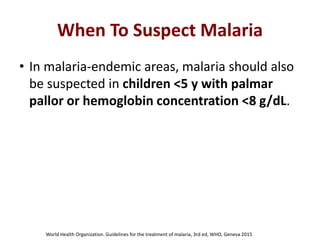
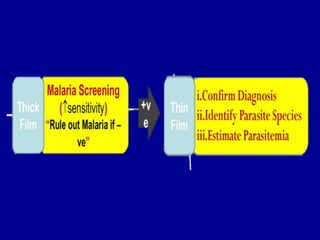
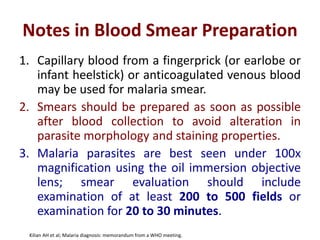
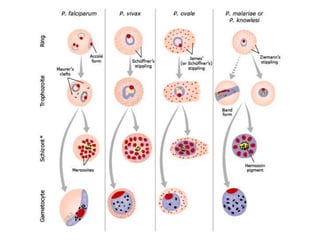
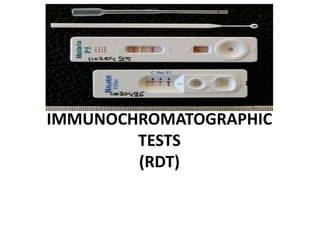
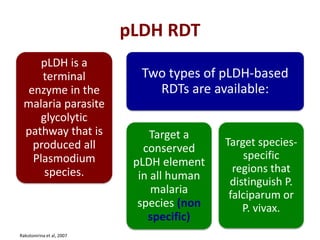
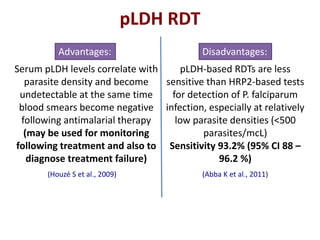
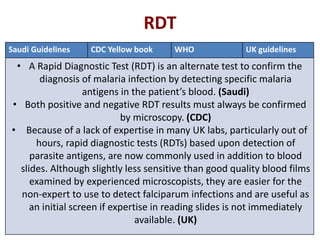
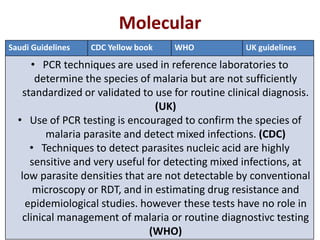
This document discusses malaria diagnostics, emphasizing the significance of accurate and timely detection for effective treatment and epidemiological purposes. It reviews various diagnostic methods, including microscopy, rapid diagnostic tests (RDTs), and molecular techniques, highlighting their advantages and limitations. The document also underlines the importance of adhering to guidelines for laboratory confirmation and monitoring of malaria cases.