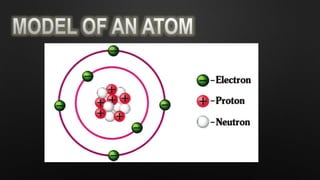
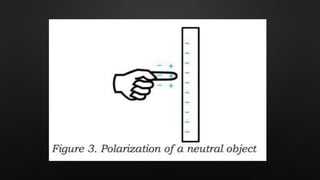
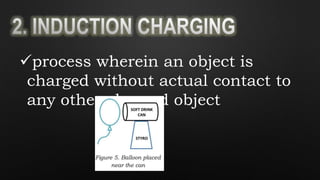
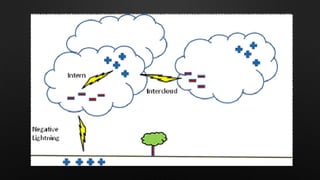
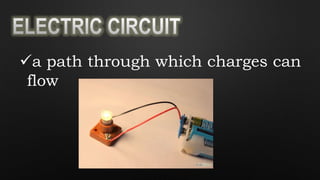
Electricity is the flow of electrons in a material. It can be transferred from one object to another, resulting in the objects having positive or negative charges depending on whether there are more protons or electrons. Like charges repel and opposite charges attract due to the electromagnetic force. Conductors allow charge to flow through them, while insulators do not.