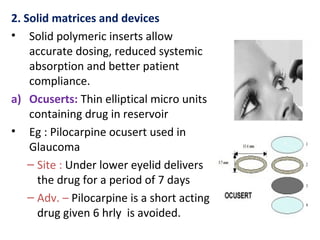
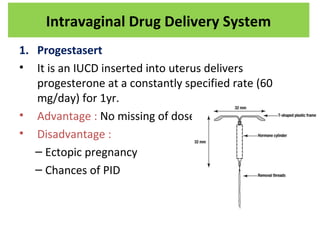
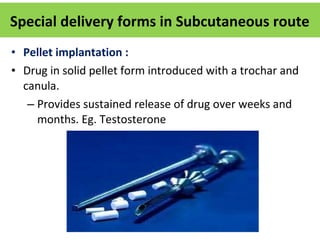
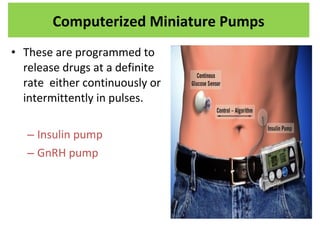
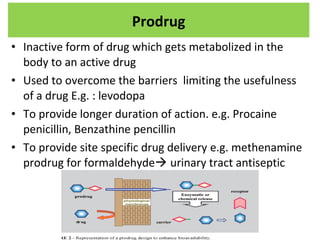
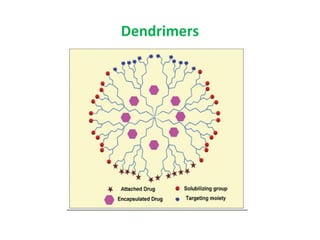
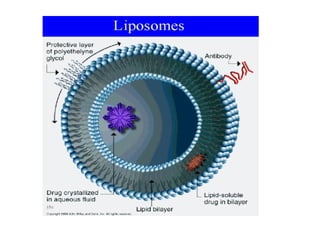
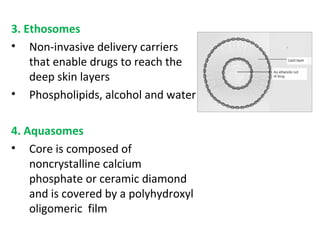
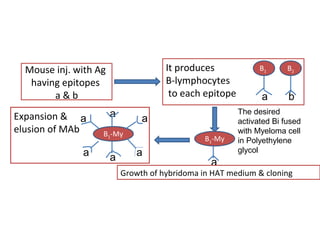
The document details various drug delivery systems, focusing on methods to improve therapeutic efficacy and patient compliance through targeted and controlled release mechanisms. It outlines types of delivery systems including oral, transdermal, nasal, and implantable methods, addressing advantages and challenges for each. Additionally, it discusses the innovation of nanoscale delivery systems, such as nanoparticles and liposomes, which enhance drug stability and effectiveness.