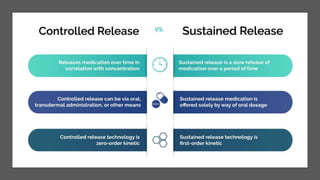
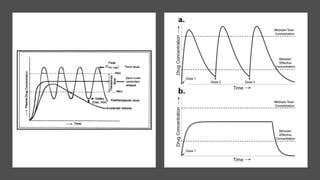
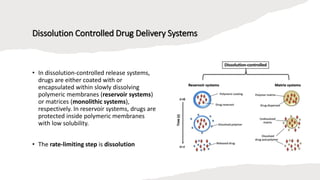
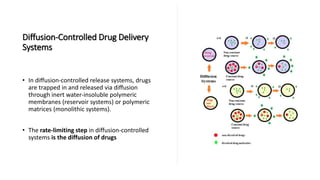
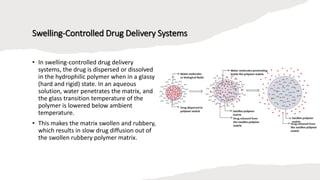
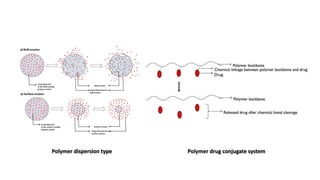
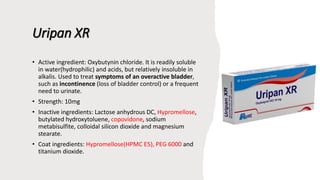
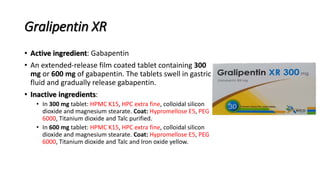
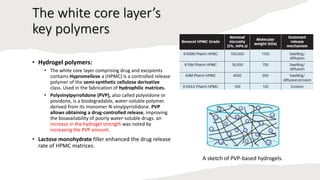
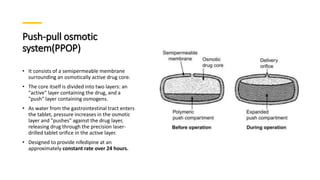
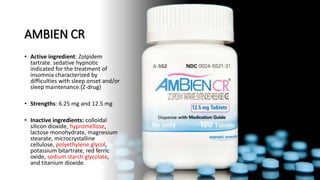
The document outlines various controlled-release drug delivery systems, emphasizing the differences between modified-release and conventional dosage forms. It describes classifications based on release mechanisms, including dissolution-controlled, diffusion-controlled, and water penetration-controlled systems, and highlights specific examples such as extended-release, delayed-release, and targeted-release products. Additionally, it provides details on various marketed drugs and their formulation technologies, showcasing how these innovations enhance patient compliance and therapeutic effectiveness.