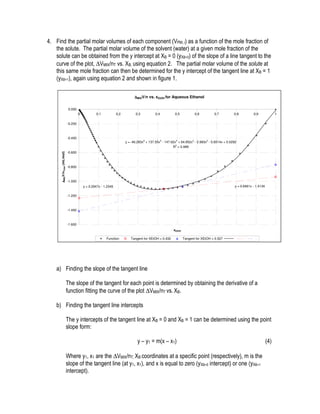
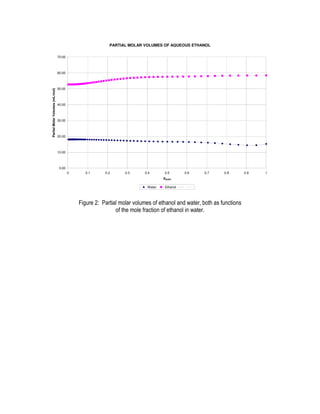
This document provides instructions for an experiment to determine the partial molar volumes of solutions using density measurements. Students will prepare solutions of varying concentrations of an alcohol in water. Density measurements will be taken and used to calculate volume, theoretical volume, and the difference between real and ideal volumes. Partial molar volumes will then be determined from the slope and intercepts of tangent lines to the plot of this difference versus mole fraction. Students will analyze the results and compare the determined partial molar volumes to literature values and trends.