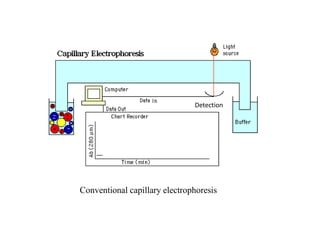
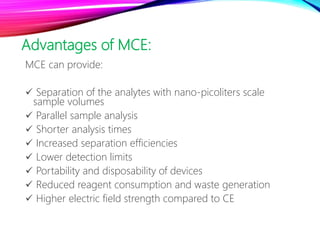
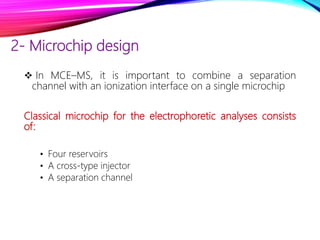
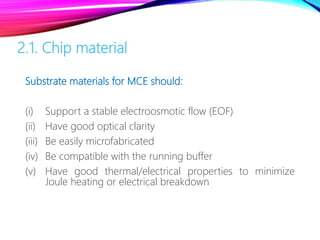
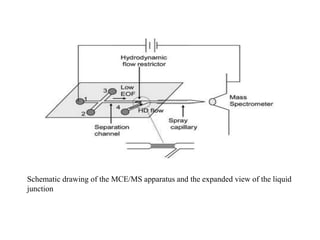
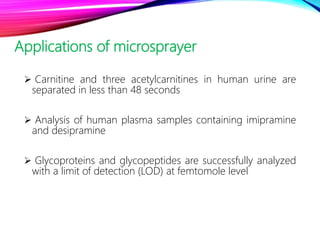
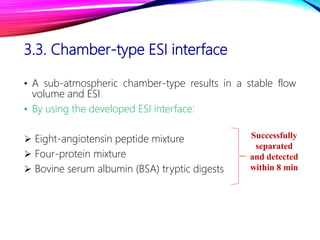
Microchip capillary electrophoresis coupled with mass spectrometry (MCE-MS) provides advantages like shorter analysis times, lower sample volumes, and higher separation efficiencies compared to conventional capillary electrophoresis. MCE uses microfabricated chips with channels and reservoirs. Effective ionization interfaces like electrospray ionization are required to couple the microchip separation to MS detection. Applications of MCE-MS include analysis of amino acids, peptides, proteins, and other biomolecules. Further development is needed to establish universal ionization methods for both micro- and macroscale analyses.