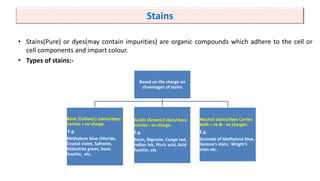
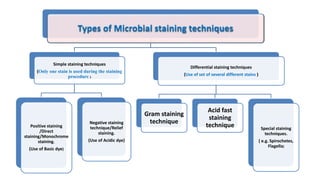
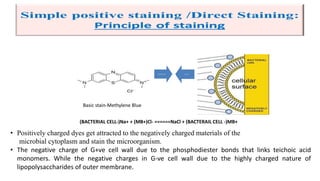
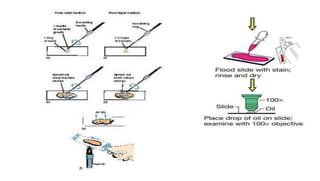
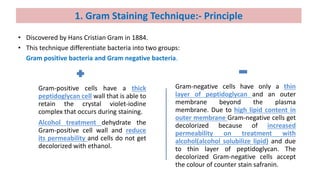
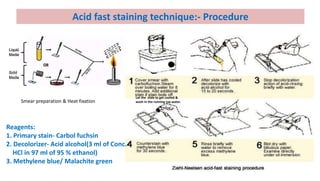
This document discusses various microbial staining techniques used to visualize microorganisms under a light microscope. It describes simple staining techniques like positive and negative staining that use single stains. It also explains differential staining techniques like Gram staining and acid-fast staining that use multiple stains to differentiate between types of microbes based on cell wall structure. Gram staining distinguishes Gram-positive from Gram-negative bacteria, while acid-fast staining identifies acid-fast bacteria like Mycobacterium that appear bright red due to their waxy cell walls. The document provides detailed procedures and observations for each staining method.