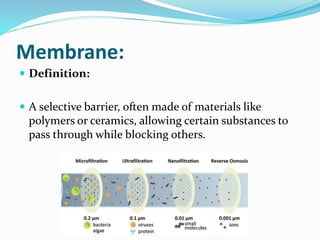
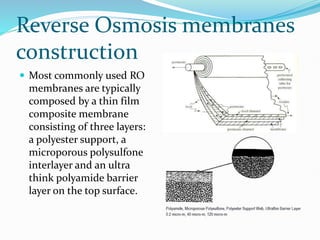
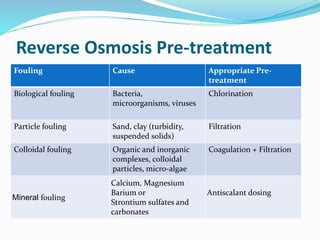
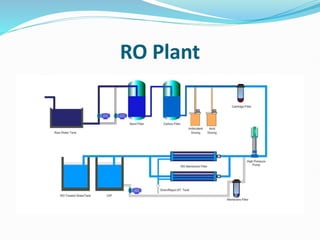
The document discusses various membrane separation processes including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis. It provides details on the working principles, pore sizes, and applications of each process. Reverse osmosis involves applying pressure to force solvent molecules like water through a dense membrane while rejecting solutes. It is highly effective for desalination and purification. The document also covers membrane construction, fouling prevention through pre-treatment, RO plant design, and the health benefits of reverse osmosis water treatment.