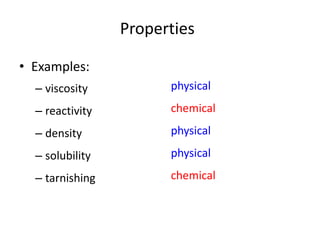
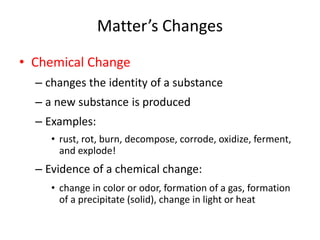
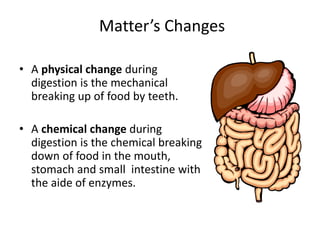
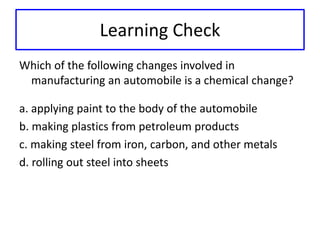
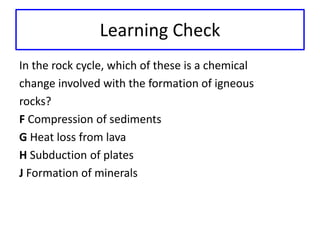
This document discusses physical and chemical properties and changes in matter. It defines physical properties as those that can be observed without changing a substance's identity, and chemical properties as those involving a change in identity to produce a new substance. Physical changes alter a substance's form or state without changing its identity, while chemical changes produce a new substance. Examples of each type of change are provided.