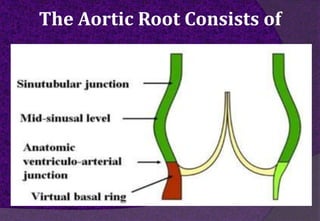
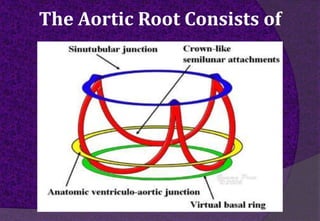
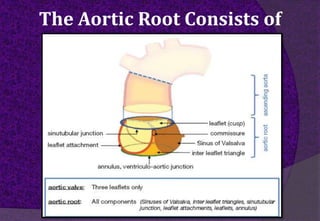
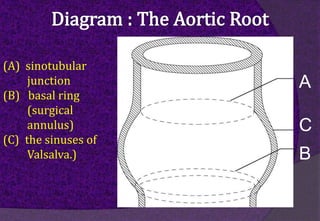
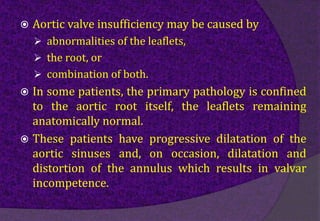
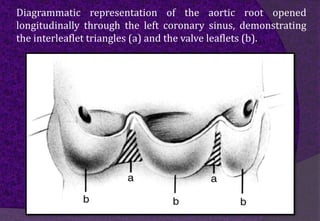
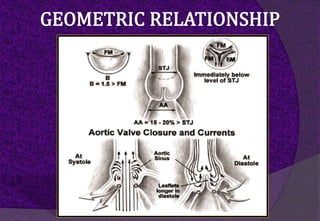
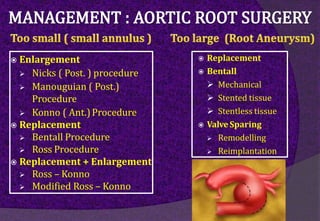
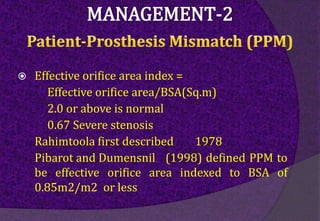
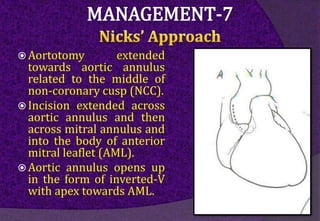
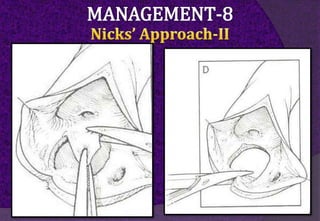
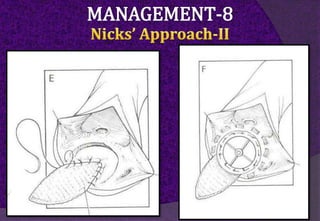
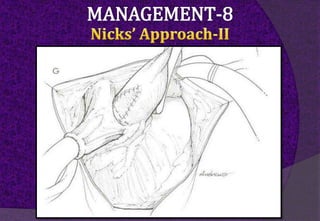
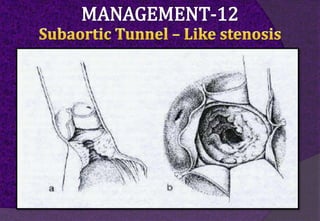
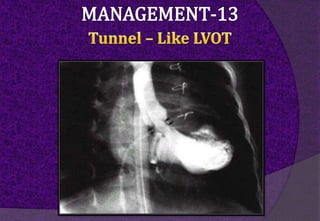
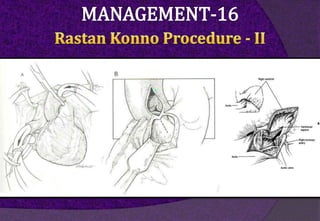
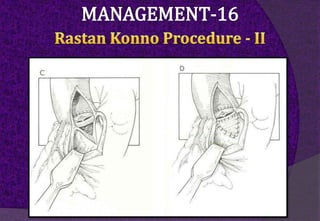
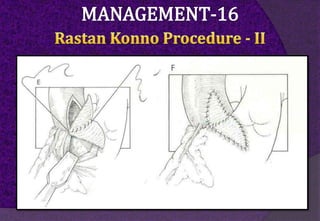
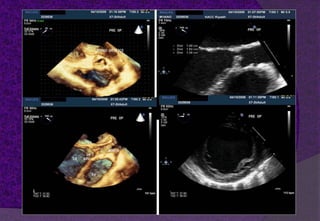
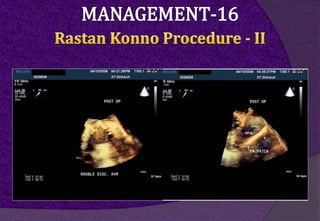
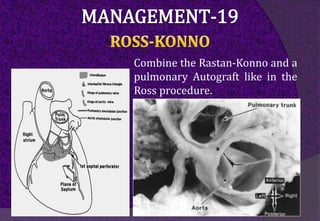
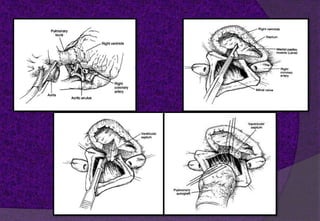
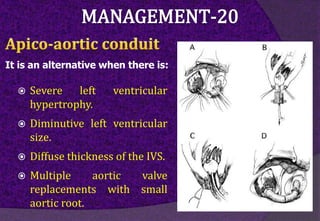
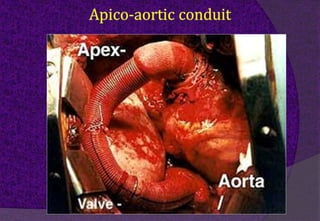
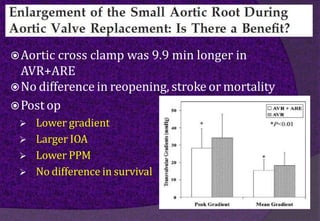
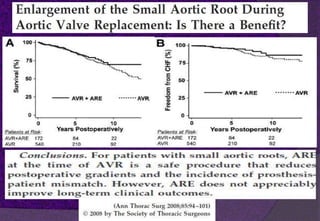
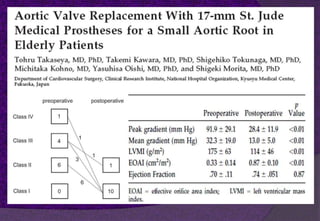
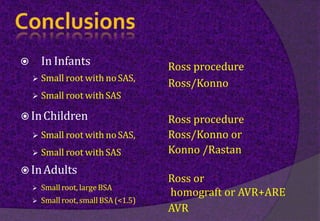
The aortic root consists of the aortic annulus, sinuses of Valsalva, and sinotubular junction. It provides support for the aortic valve leaflets and connects the left ventricle to the ascending aorta. Abnormalities of the aortic root can cause aortic insufficiency. Surgical techniques for addressing aortic root pathology include replacement using a valve conduit or autograft, as well as techniques to enlarge the annulus such as the Nicks and Manouguian procedures. The choice of technique depends on factors like patient age and anatomy.