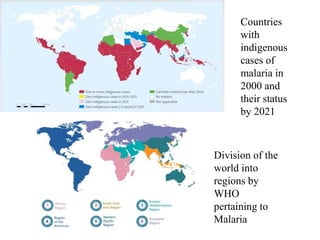
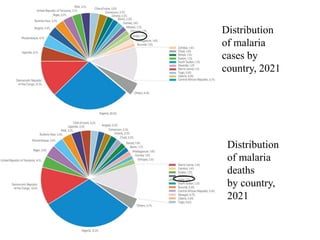
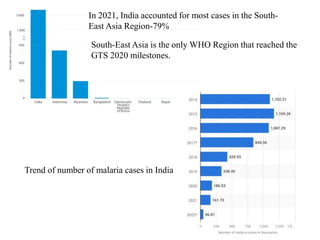
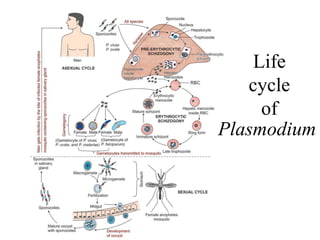
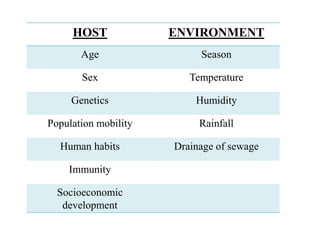
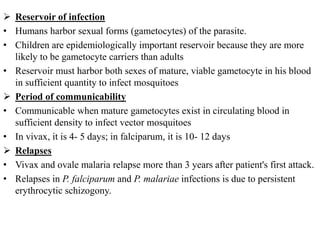
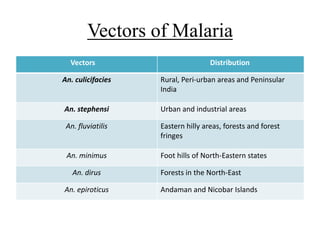
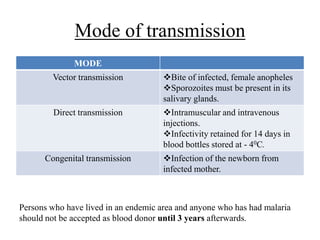
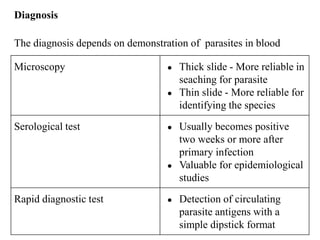
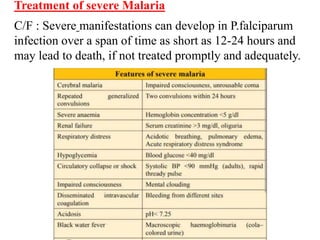
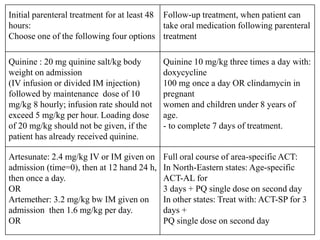
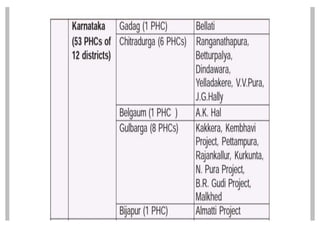
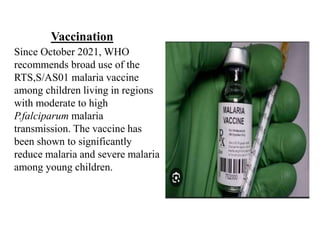
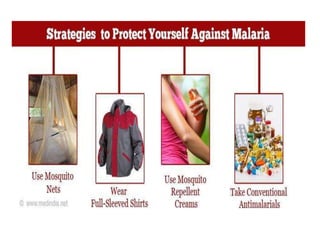
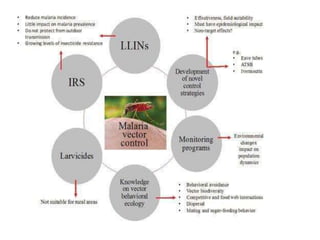
Malaria is caused by a protozoan parasite transmitted through the bites of infected Anopheles mosquitoes. In 2021, there were an estimated 247 million malaria cases globally, with India accounting for 79% of cases in the Southeast Asia region. Malaria symptoms include fevers that occur in cycles corresponding to the asexual reproduction cycle of the parasite in the human host. Diagnosis is typically made by microscopy identification of the parasite in blood smears, with treatment depending on the identified Plasmodium species. For uncomplicated malaria, chloroquine and ACTs are recommended, while severe malaria requires initial parenteral treatment with quinine or artesunate. Prevention focuses on vector control and the use of insecticide











![Treatment failure
After treatment patient is considered cured if he/she does not have fever or
parasitaemia till day 28th
Early treatment failure [ETF]
● Development of danger signs or
severe Malaria on Day 1, 2 or 3
in the presence of parasitaemia
● Parasitaemia on Day 2 higher
than on Day 0, irrespective of
axillary temperature
● Parasitaemia on day 3 with
axillary temperature >37.5 c
Late parasitological failure
[LPF]
● Presence of parasitaemia on any
day between day 7 and day 28
with axillary temperature <37.5c
in patients who did not
previously meet any of the
criteria of early treatment failure](https://image.slidesharecdn.com/malariafinal2-240208075556-09abeaab/85/Malaria-vector-and-management-final-pptx-33-320.jpg)



















![REFERENCES
1. CodeHunk. National ANTI-MALARIA programme [Internet].
[cited 2023 Jun 27]. Available from:
http://www.nihfw.org/NationalHealthProgramme/NATIONALANTI
_MALARIAPROGRAMME.html
2. World Malaria Report 2022 [Internet]. World Health
Organization; [cited 2023 Jun 27]. Available from:
https://www.who.int/news-room/fact-sheets/detail/malaria
3. Park K. Epidemiology of communicable diseases. In: Park’s
textbook of Preventive and Social Medicine. 26th edition ed. India:
Bhanot Publishers; 2022.](https://image.slidesharecdn.com/malariafinal2-240208075556-09abeaab/85/Malaria-vector-and-management-final-pptx-59-320.jpg)