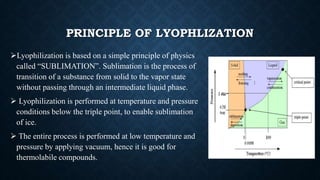
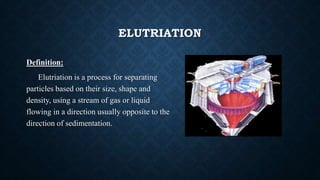
The document discusses lyophilization and elutriation. Lyophilization, or freeze drying, is a process that removes water from a frozen product under vacuum, allowing ice to change directly to vapor without passing through liquid. It involves freezing, primary drying to remove 98-99% water, and secondary drying to remove remaining bound water. Elutriation separates particles based on size, shape and density using an opposing gas or liquid stream, allowing finer particles to move upward while larger ones sediment downward.