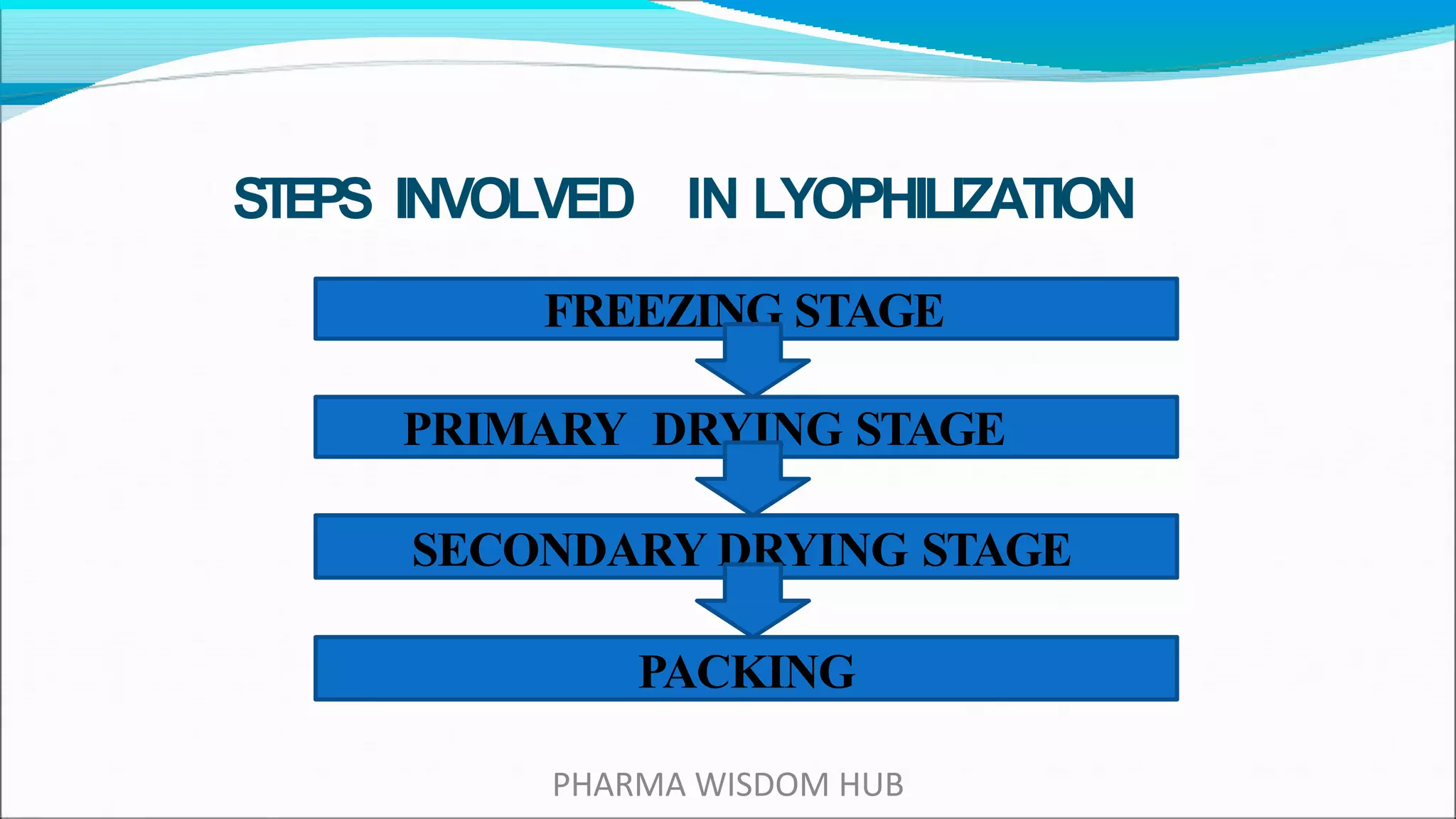
Lyophilization, or freeze drying, is a process that stabilizes substances by first freezing them and then removing the solvent through sublimation and desorption, mainly to preserve biological activity and extend shelf life. Developed during World War II, this technique is crucial for thermolabile compounds and involves steps like freezing, vacuum application, heating, and condensation. While it has advantages like maintaining sterility and precise fill weight control, disadvantages include potential damage to biological molecules and high costs.