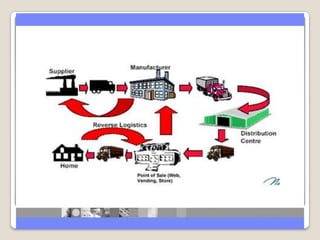
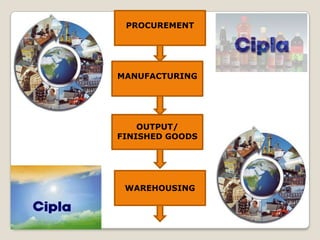
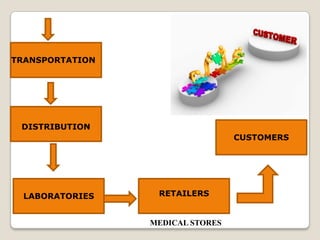
The document summarizes the logistics process of pharmacy companies like Cipla. It discusses how Cipla manages the flow of resources from suppliers through manufacturing, warehousing, transportation and distribution to retailers and customers. Key parts of Cipla's supply chain include procuring materials from suppliers, manufacturing drugs, storing and transporting finished goods, and distributing to medical stores and retailers from where customers obtain the drugs.