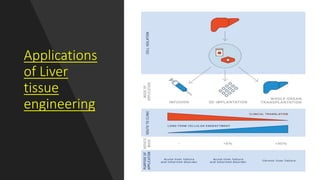
The document discusses liver tissue engineering and technologies for implantable liver therapies. It describes:
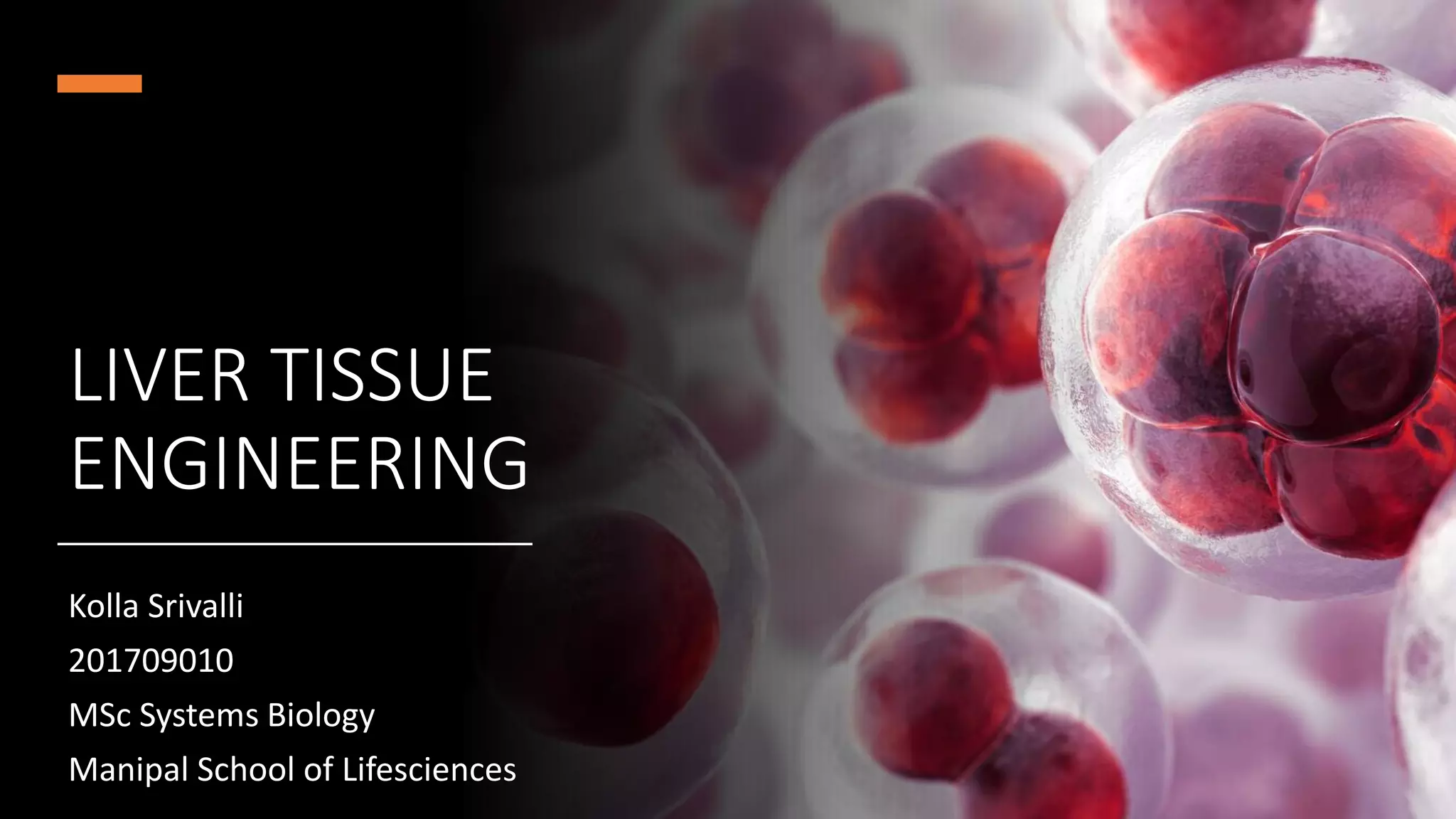
1. The types of cells in the liver and their functions.
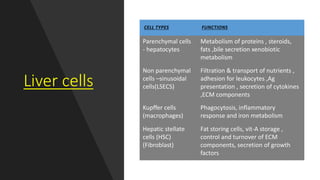
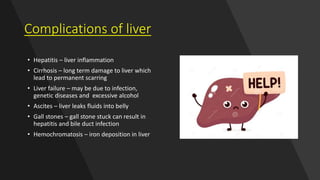
2. Complications that can result from liver damage like cirrhosis and failure.
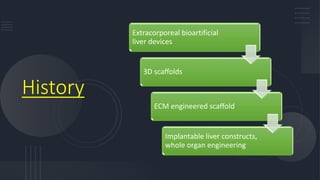
3. The history and development of implantable technologies including cell encapsulation, 3D printing, scaffolds, and decellularization/recellularization techniques to engineer liver tissue for transplantation.
4. Applications include using decellularized liver scaffolds that can be repopulated with cells to create functional liver tissue for transplantation or models for drug testing.