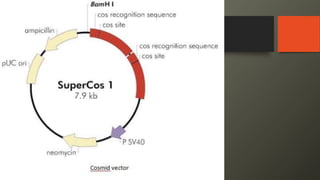
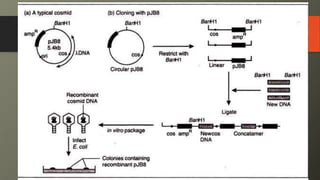
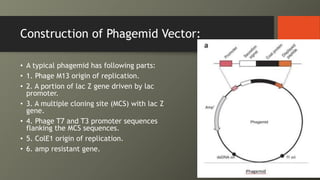
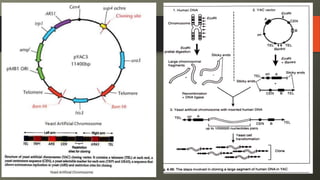
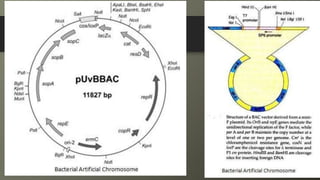
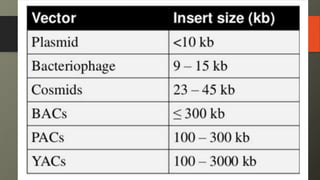
Cosmids are hybrid vectors that combine features of bacteriophages and plasmids. They can clone large DNA fragments of 25-45 kb. Cosmids contain cos sites that allow packaging of the foreign DNA by lambda phage proteins. Phagemids contain both phage and plasmid replication origins, allowing replication as a plasmid and packaging as single-stranded DNA in phage particles. Bacterial artificial chromosomes (BACs) are derived from bacterial plasmids and can clone inserts of 150-350 kb in E. coli. They are more stable than yeast artificial chromosomes (YACs) but can hold smaller inserts. YACs can accommodate megabase-sized inserts in yeast but are prone to rearrange