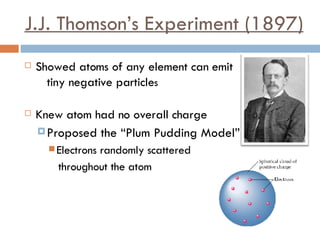
1. J.J. Thomson's experiment in 1897 showed that atoms emit tiny negative particles, known as electrons, and are electrically neutral overall, leading him to propose the "plum pudding" model of the atom.
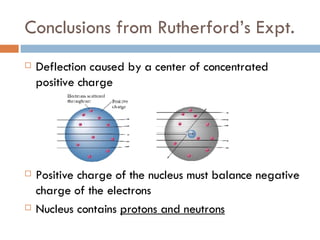
2. In 1910, Rutherford discovered that some alpha particles were deflected when fired at a thin metal sheet, surprising him and leading to the conclusion that atoms have a small, dense, positively charged nucleus.
3. Isotopes are atoms with the same number of protons but different numbers of neutrons. The atomic number is the number of protons, the mass number is the sum of protons and neutrons, and the relative atomic mass is the weighted average of