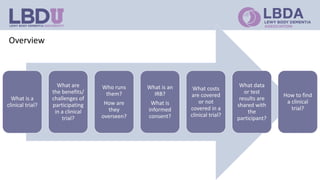
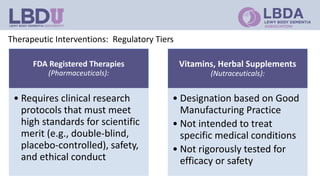
Clinical trials are research studies that test new medical treatments and are conducted according to a research plan called a protocol. A clinical trial has a principal investigator and research team who are responsible for overseeing the trial. Participants must meet eligibility criteria and provide informed consent to enroll. The trial follows a treatment plan, which may involve experimental treatments, standard treatments, or placebos. Participants are monitored by an Institutional Review Board to ensure safety and ethical treatment. Clinical trials provide information about new treatments and help advance medical knowledge, but may not directly benefit participants.