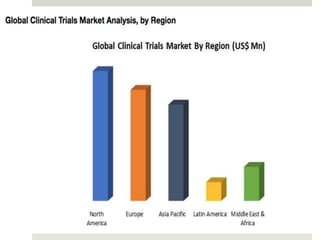
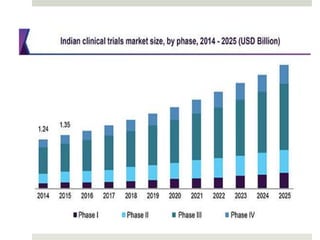
Clinical research involves scientific studies that use human volunteers to improve healthcare practices, focusing on both observational and interventional trials. A clinical trial systematically evaluates new drugs or therapies to determine their safety and efficacy while following a defined protocol aimed at enhancing standard care. Participation in such trials offers potential benefits such as access to new treatments and expert care, though it also carries risks including side effects and increased demands on participants' time.