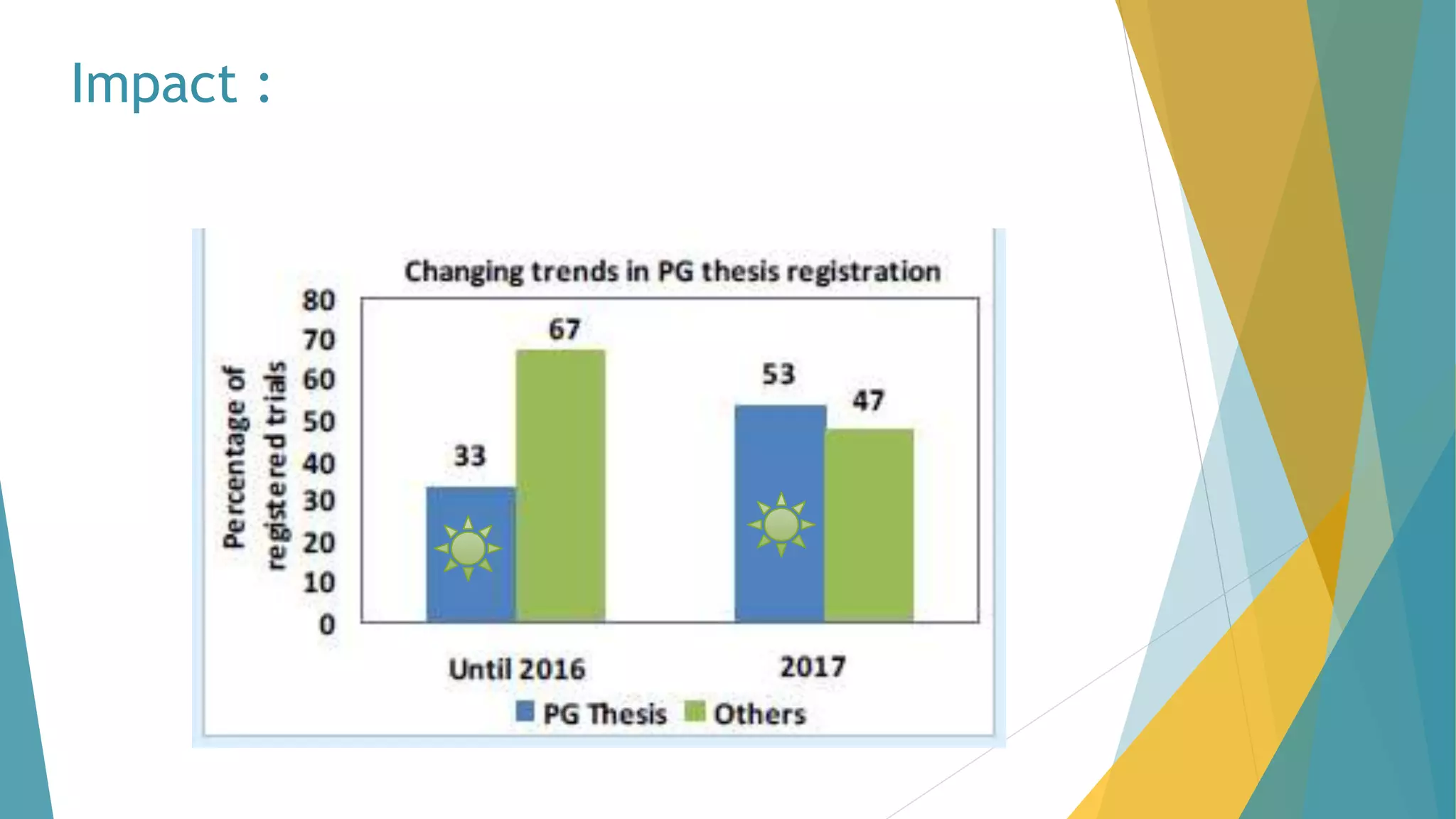
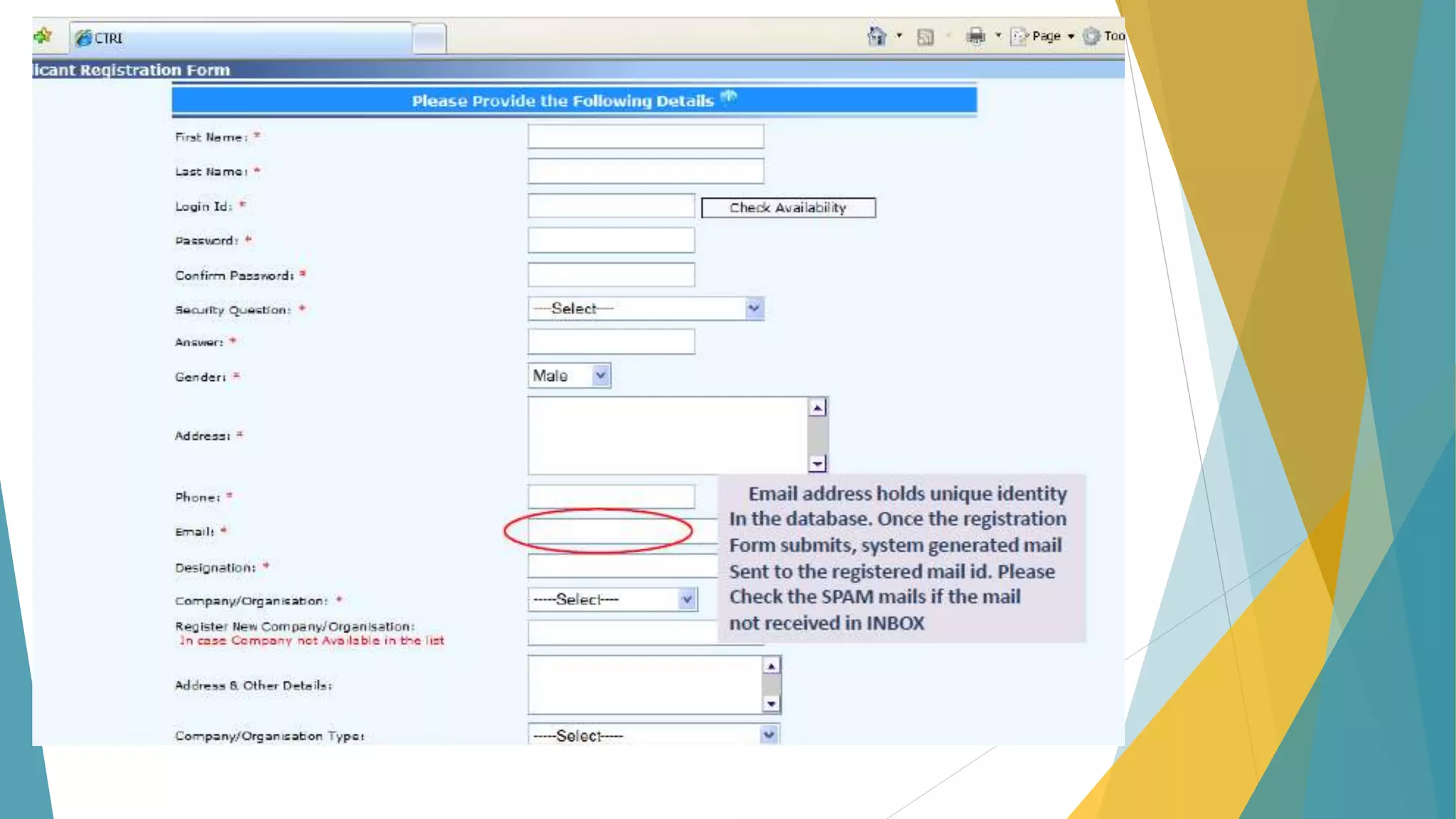
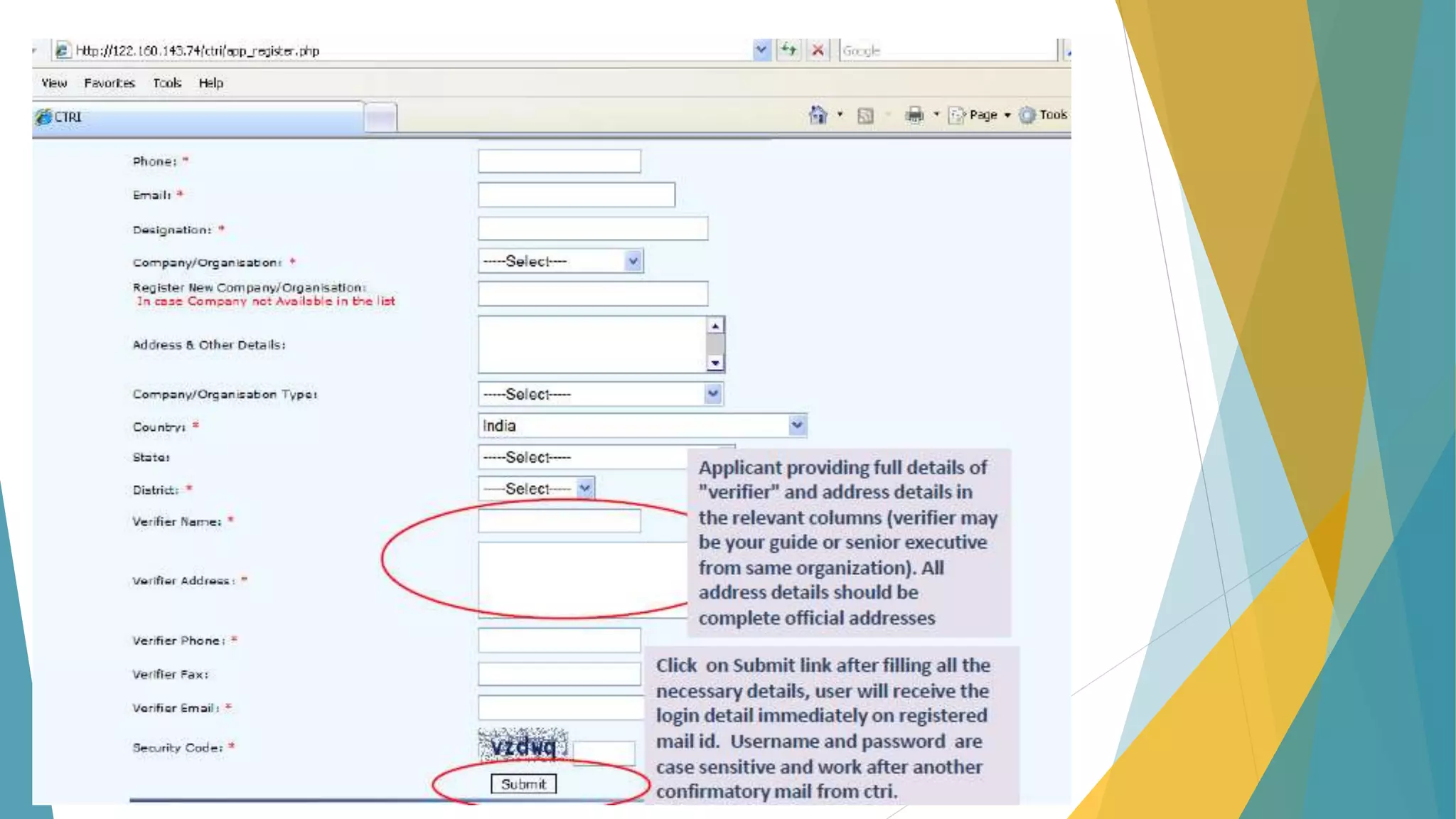
The document discusses the importance of clinical trial registration to combat selective publication bias and enhance transparency in clinical research. It details the International Clinical Trials Registry Platform (ICTRP) and the Clinical Trial Registry - India (CTRI), emphasizing their roles in ensuring all clinical trials are publicly registered and accountable. Additionally, it outlines the registration process, requirements, and the benefits of maintaining a transparent trial record for patients and researchers.