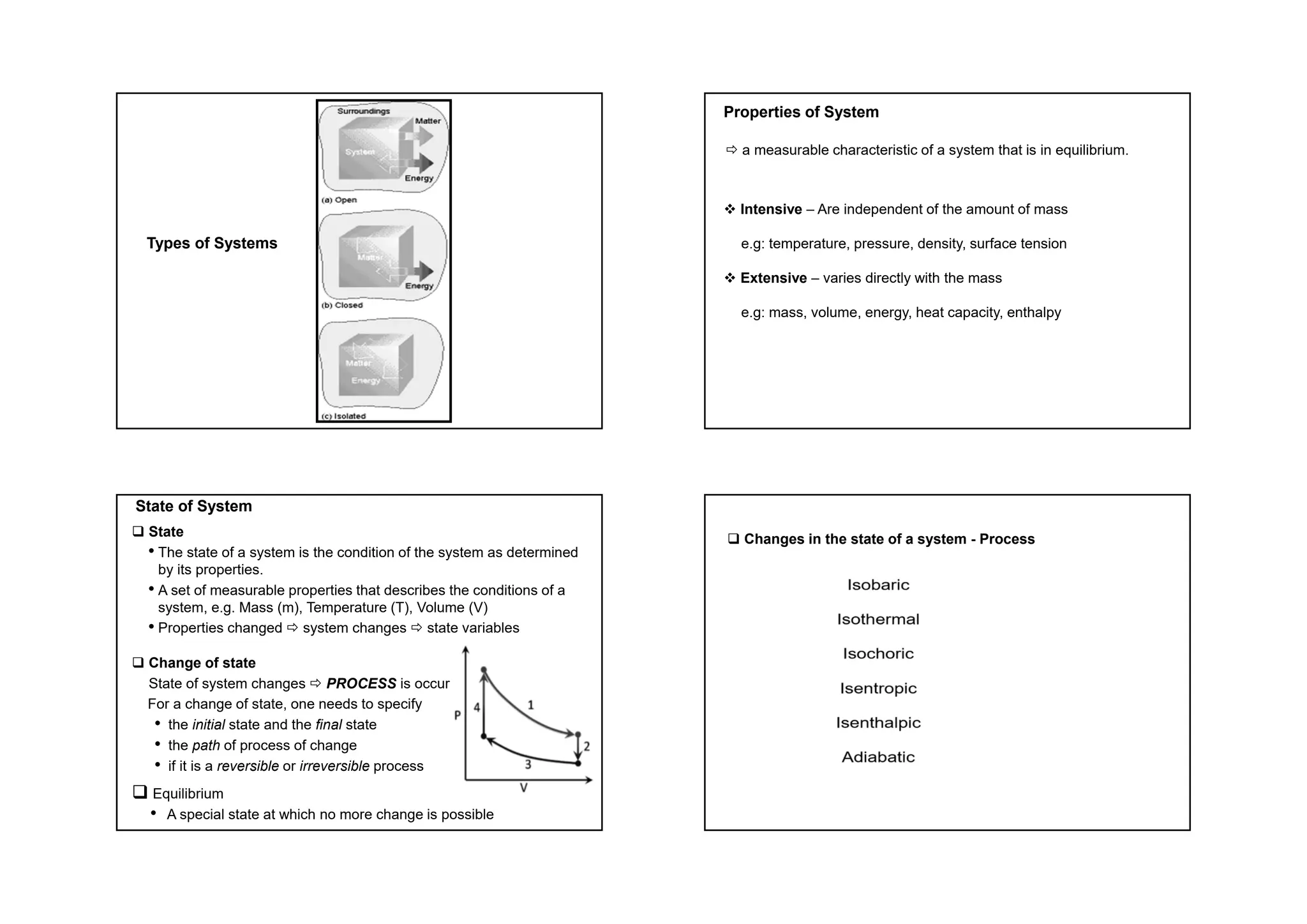
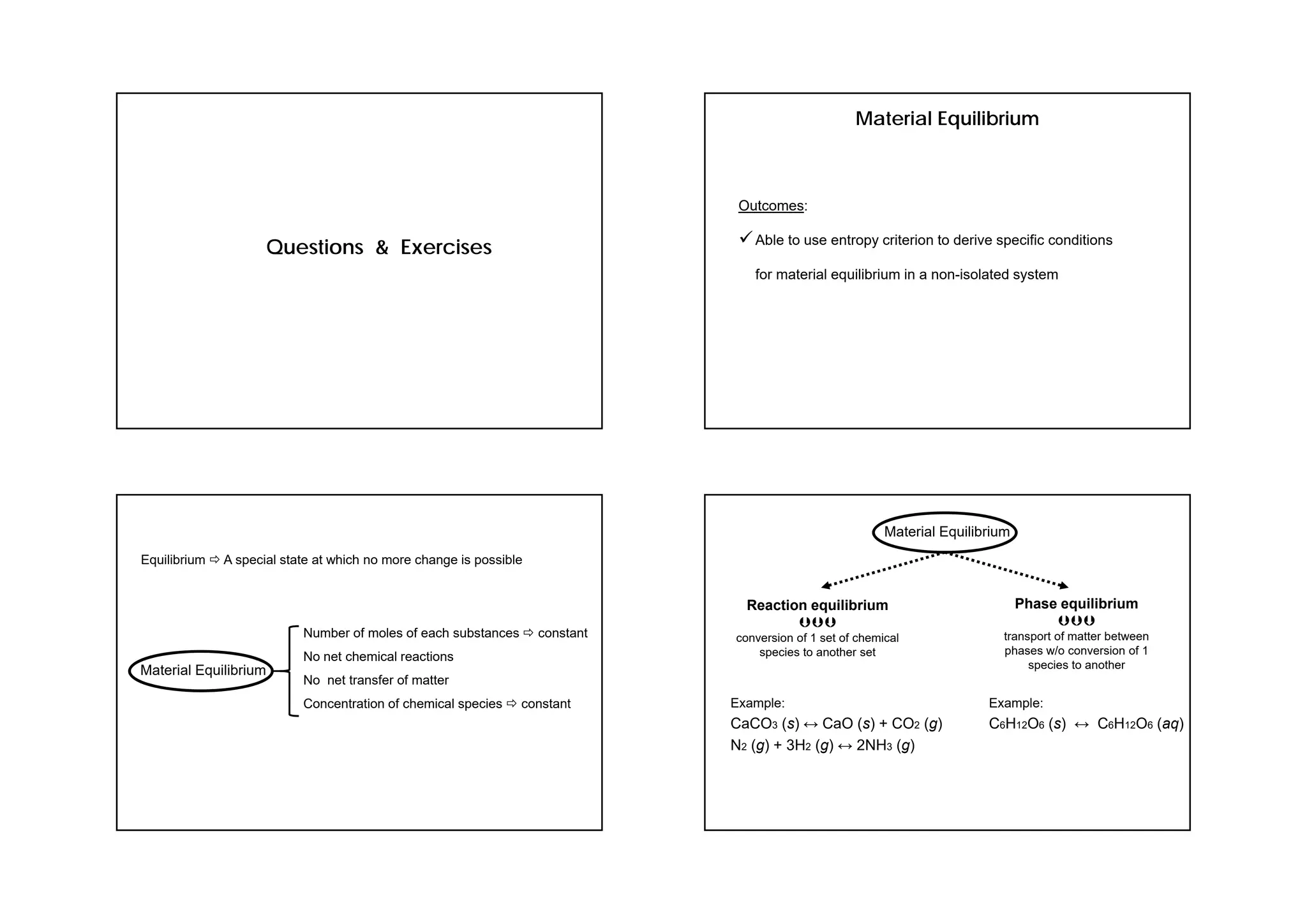
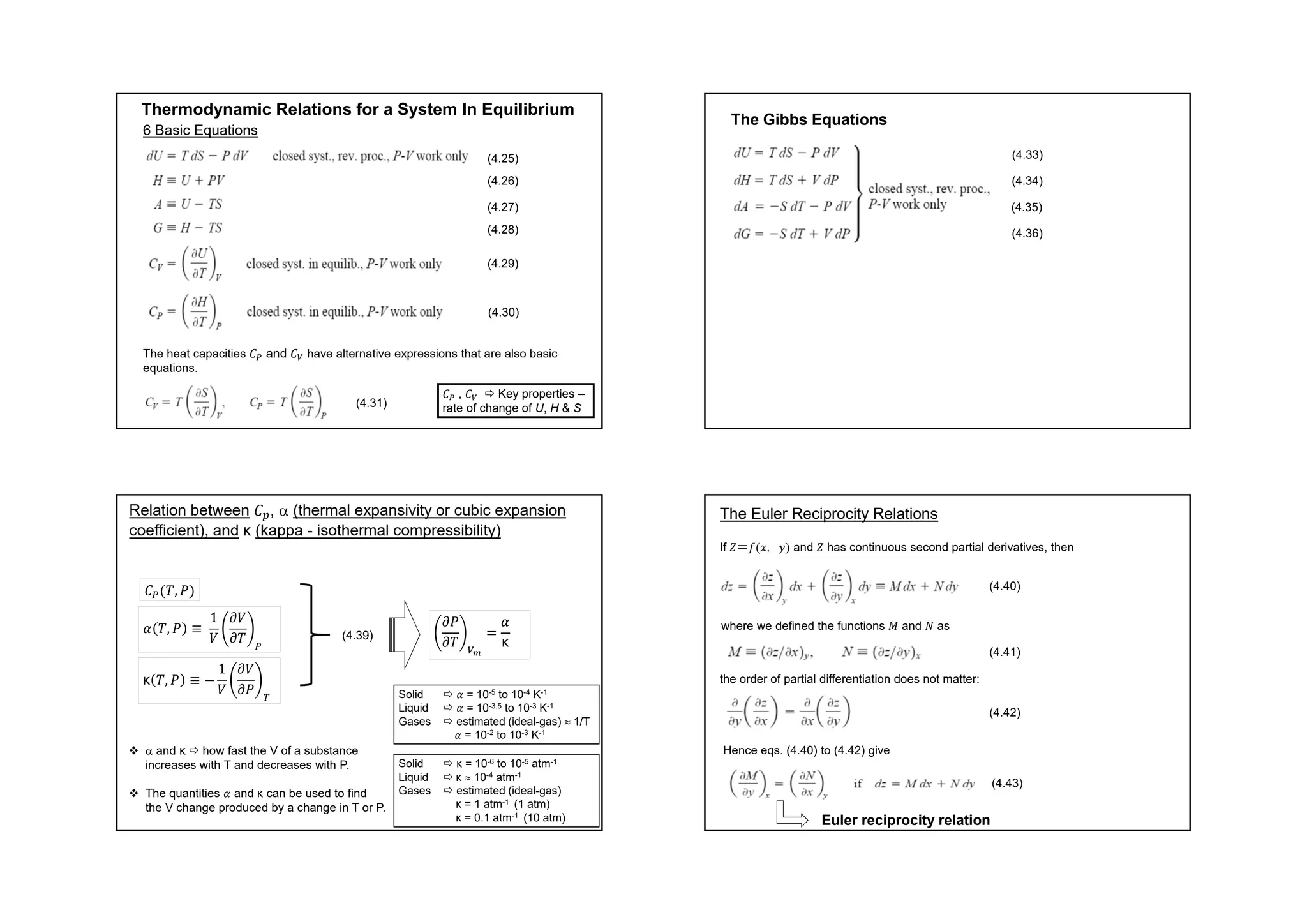
This document provides information about a physical chemistry course for the 2018/2019 semester. It includes:
- Details about the course instructors and their contact information.
- An outline of the course content including topics to be covered, references, objectives, outcomes and assessment details.
- The course is worth 3 credit units and students will be evaluated based on exams (60%), coursework (40%) including tests, assignments and quizzes.
- Class times and locations are provided, with notes that attendance is mandatory or students may be prevented from sitting exams.