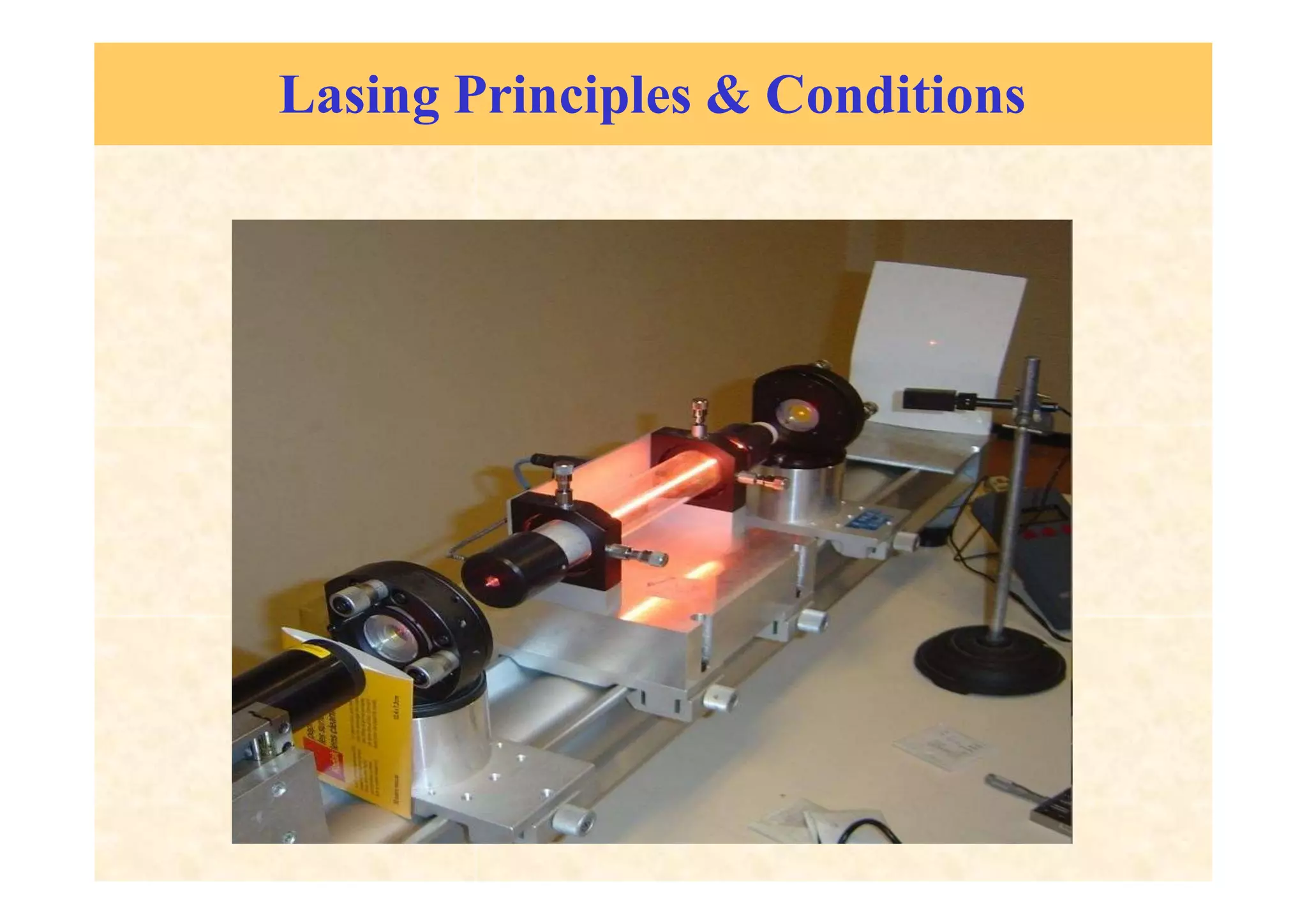
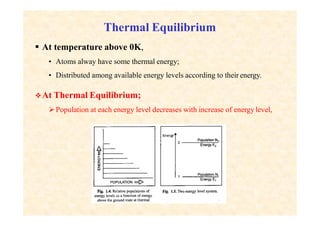
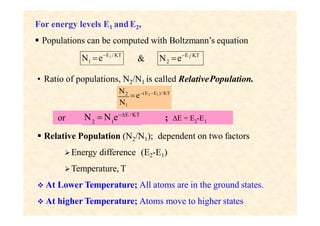
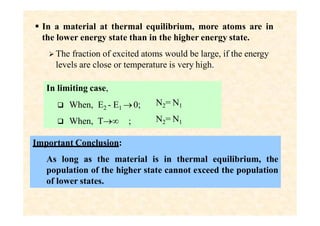
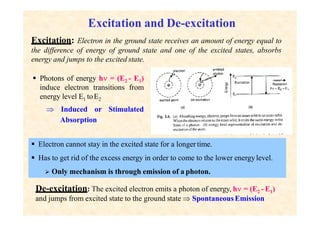
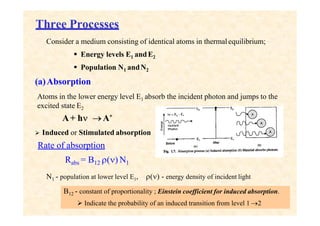
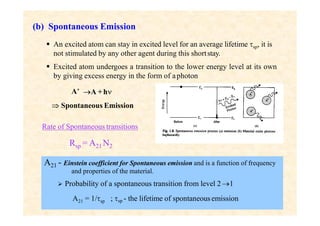
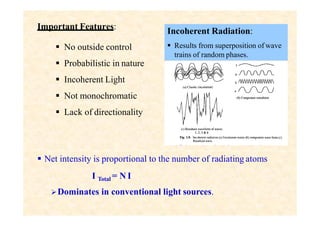
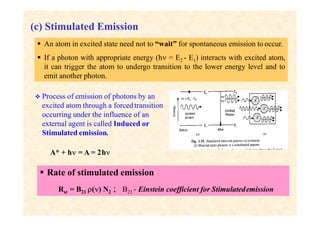
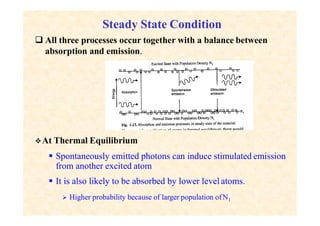
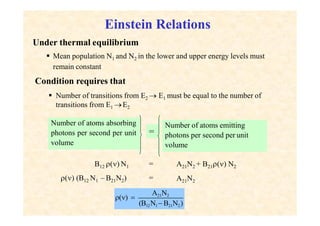
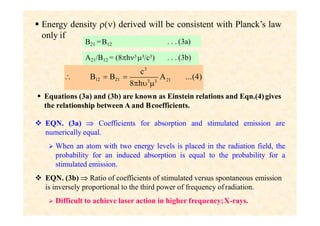
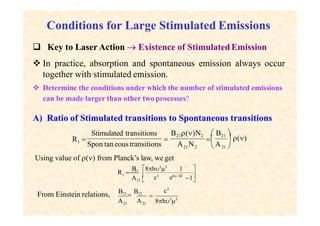
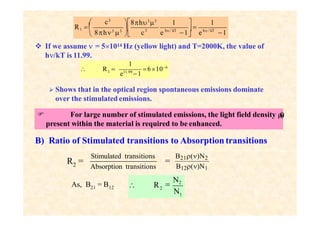
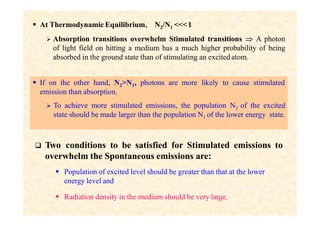
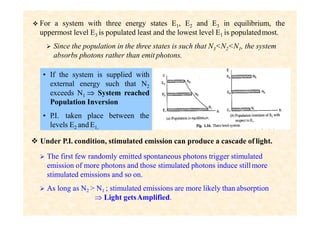
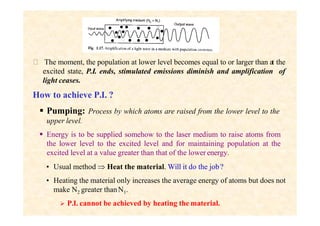
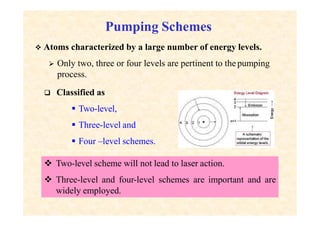
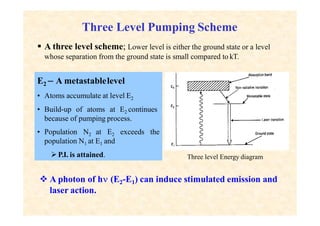
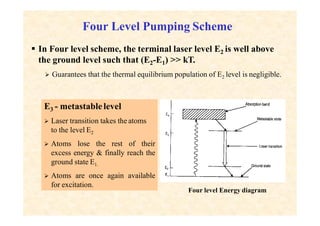
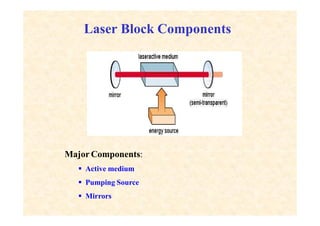
This document discusses the principles of lasing and population inversion. It explains that atoms have discrete energy levels and are typically in the lowest energy or ground state. For lasing, a population inversion is needed where more atoms are in an excited state than the ground state. This can be achieved by optical or electrical pumping methods. Optical pumping uses light to selectively excite atoms to higher energy levels, while electrical pumping passes current through gas lasers. Stimulated emission of photons can then occur, leading to amplification of light and lasing.