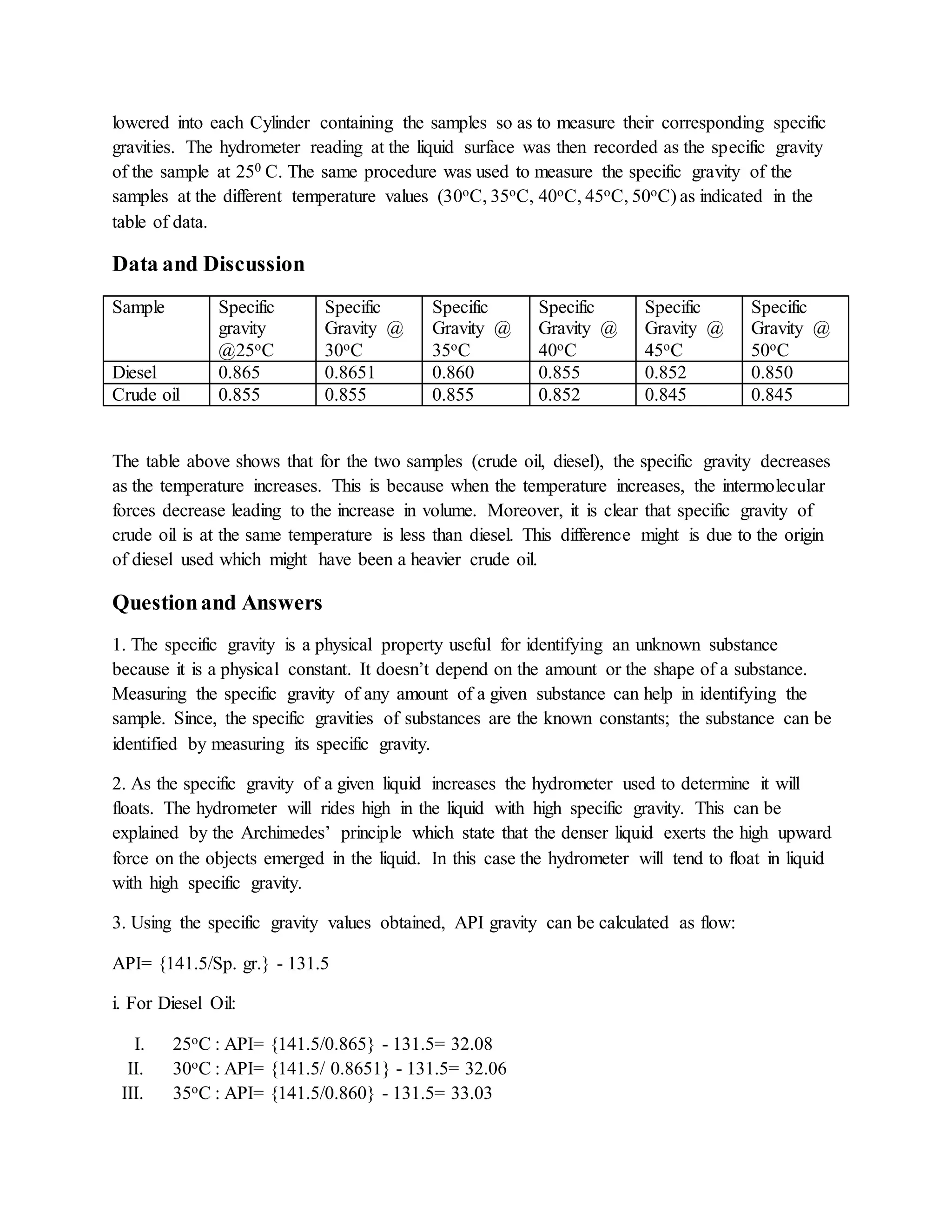
The document details an experiment aimed at determining the specific gravity of crude oil and diesel using a hydrometer at various temperatures. The findings indicate that specific gravity decreases as temperature rises, with crude oil consistently being denser than diesel. Additionally, it concludes that API gravity increases as specific gravity decreases, highlighting the relationship between temperature, specific density, and the properties of these petroleum liquids.