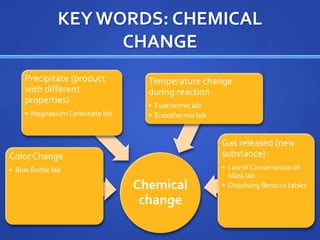
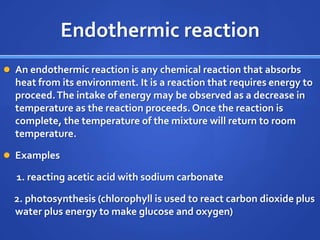
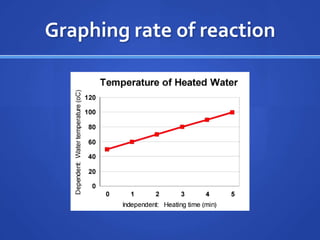
This document provides study tips and information for preparing for a science lab test. It recommends practicing with lab tools and making graphs using data tables. Key science words and previous lab reports should be reviewed. Other study aids include online quizzing resources and creating your own practice quizzes. Key concepts covered include chemical and physical changes, endothermic and exothermic reactions, and the law of conservation of mass. Examples are given for each concept.