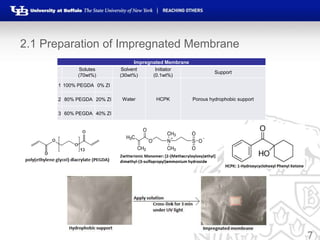
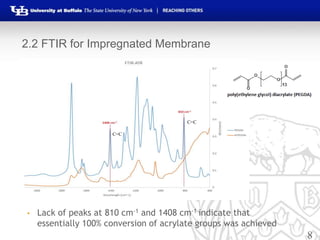
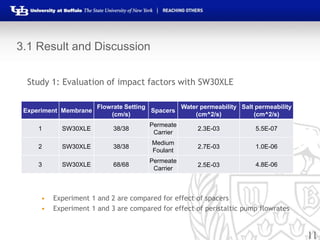
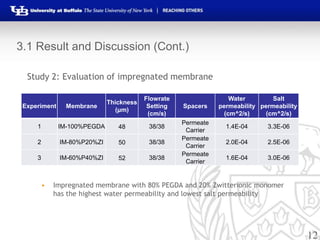
This document summarizes research on developing forward osmosis membranes for water purification. It presents two studies: 1) evaluating the impact of spacers and flow rates on an SW30XLE membrane, finding that spacers with higher pore size provide higher water and salt permeability; and 2) evaluating three impregnated membranes with different compositions, finding the membrane with 80% PEGDA and 20% zwitterionic monomer had the highest water permeability and lowest salt permeability. The document outlines the experimental methods, results, and conclusions from both studies and development of impregnated forward osmosis membranes for water purification applications.