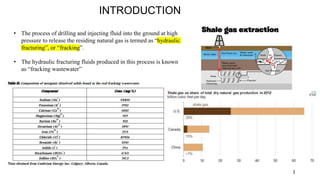
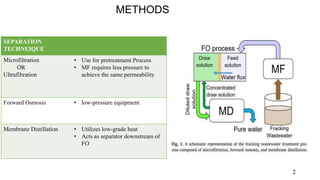
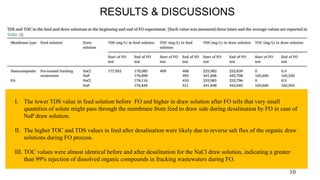
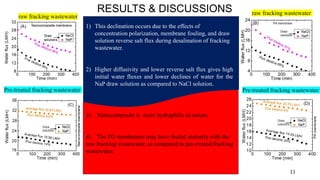
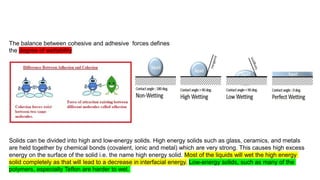
The document presents a paper on the feasibility of a hybrid membrane-based process (MF-FO-MD) for treating fracking wastewater, focusing on methods including microfiltration (MF), forward osmosis (FO), and membrane distillation (MD). Results indicate that a nanocomposite microfiltration membrane outperforms a polysulfone membrane in terms of water permeability and fouling resistance, demonstrating a potential for high salinity waste treatment. The findings suggest that this novel combined process could effectively manage the challenges associated with fracking wastewater treatment.