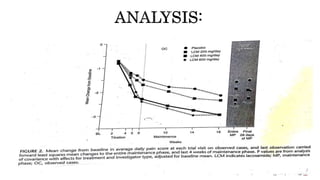
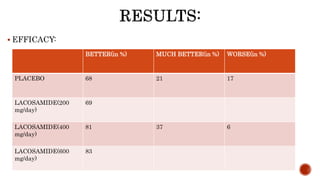
This study evaluated the efficacy and safety of lacosamide in treating diabetic neuropathic pain through an 18-week double-blind trial of 370 patients with doses of 200 mg/day, 400 mg/day, and 600 mg/day compared to a placebo. The 400 mg/day dose was found to significantly improve pain scores over the placebo and had the optimal balance of efficacy and side effects. Common side effects included dizziness, tremor and headache. While lacosamide showed potential for treating diabetic neuropathic pain, the study period was short and the 600 mg/day group had a high withdrawal rate due to adverse events.