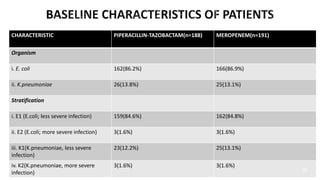
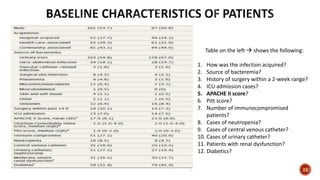
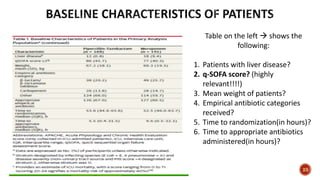
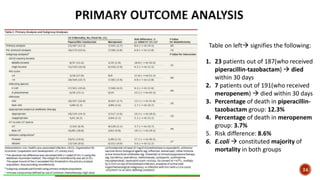
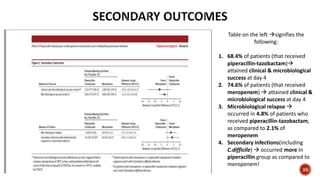
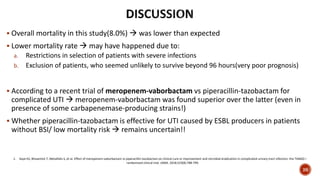
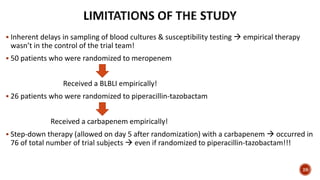
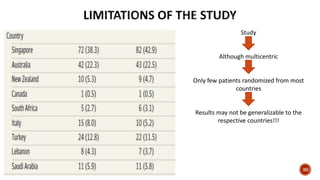
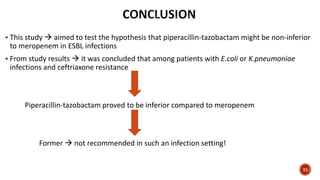
This document discusses a randomized clinical trial comparing the effectiveness of piperacillin-tazobactam and meropenem in treating bloodstream infections caused by ceftriaxone-resistant E. coli and Klebsiella pneumoniae. The study found that piperacillin-tazobactam was associated with higher mortality and lower clinical success rates, making it inferior to meropenem in this context. The results suggest that meropenem remains the preferred treatment for ESBL infections, highlighting concerns over increased antibiotic resistance.



![ Gram (-ve) bacteria produce ESBL enzymes
Contribute to significant health concern globally!
According to US CDC estimates from 2011
ESBL-producing organisms
Contributed to 26,000 infections & 1,700 deaths annually!
1. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159-166. [Crossref]
2. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. Washington, DC: US Department of Health and Human Services; 2013. [Crossref] 4](https://image.slidesharecdn.com/journalclubpresentation-20-190320185447/85/Journal-club-presentation-by-RxVichuZ-4-320.jpg)
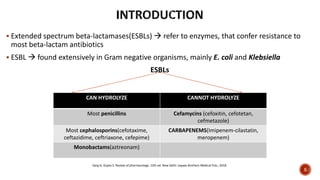
![ ESBL-producers alarmingly prevalent in both community & health-care settings
Although carbapenems are DOC* for ESBL infections
Increased use of former
Can result in
Ineffectuality in G(-ve) bacilli!
In such situations usage of alternative carbapenem-sparing agents (eg. Piperacillin-
tazobactam)
Might be an effective option to reduce global burden on carbapenems
*Drug of choice
1. Doi Y, Park YS, Rivera JI, et al. Communityassociated extended-spectrum β-lactamase producing Escherichia coli infection in the United States. Clin Infect Dis. 2013;56(5):641-648[Crossref]
2. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657-686[Crossref]
3. Chang H-J, Hsu P-C, Yang C-C, et al. Risk factors and outcomes of carbapenem-non susceptible Escherichia coli bacteremia: a matched case-control study. J Microbiol Immunol Infect. 2011;44(2):125-130[Crossref]
6](https://image.slidesharecdn.com/journalclubpresentation-20-190320185447/85/Journal-club-presentation-by-RxVichuZ-6-320.jpg)
![Although BLBLIs* are documented to be effective in ESBL infections
Results are rather controversial!
Thus
This study aims to hypothesize if usage of a carbapenem-sparing agent(piperacillin-tazobactam)
Would
Prove to be non-inferior to a carbapenem(meropenem) for ESBL infections.
*Beta-lactam-beta-lactamase inhibitor
1. Harris PN, Tambyah PA, Paterson DL. β-lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic
options? Lancet Infect Dis. 2015;15(4):475-485 [Crossref]
2. Tamma PD, Rodriguez-Bano J. The use of noncarbapenem β-lactams for the treatment of extended-spectrum β-lactamase infections. Clin Infect Dis. 2017;64(7):972-980 [Crossref]
3. Tamma PD, Han JH, Rock C, et al; Antibacterial Resistance Leadership Group. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase
bacteremia Clin Infect Dis. 2015;60(9):1319-1325. [Crossref]
7](https://image.slidesharecdn.com/journalclubpresentation-20-190320185447/85/Journal-club-presentation-by-RxVichuZ-7-320.jpg)