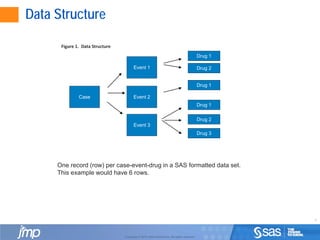
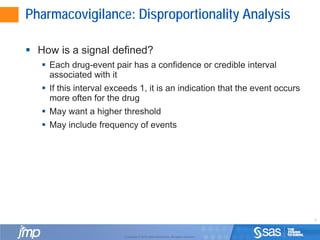
This document discusses approaches for analyzing spontaneously reported adverse events from post-market drug surveillance. It describes how clinical trials provide an incomplete safety profile and how data from post-market use can reveal rare or long-term safety issues. Statistical methods like disproportionality analysis are used to detect unexpected drug-event combinations in spontaneous reporting data by comparing the frequency of reports for a drug-event pair to what would be expected based on overall reporting rates. Stratifying the data by patient characteristics can improve the accuracy of these analyses. Signals of disproportionate reporting are defined as drug-event pairs where the confidence or credible interval for their association exceeds a threshold value.