Embed presentation
Downloaded 44 times






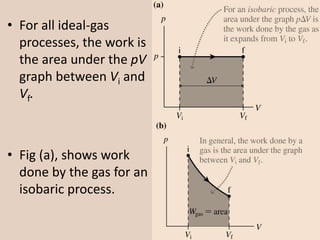



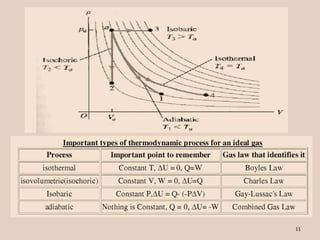


The document discusses the isobaric (constant pressure) process in thermodynamics, highlighting that pressure remains constant while the heat transferred to the system does work and alters internal energy. It explains how this process is represented on a PV diagram and mentions the relationship between pressure, volume, and temperature for ideal gases. The presentation concludes with a boundary work equation relevant to constant pressure conditions.











