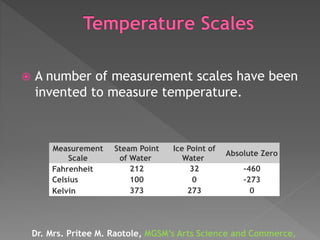
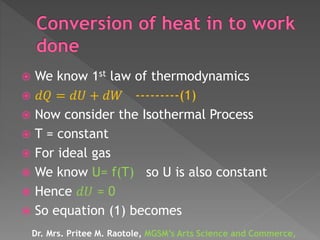
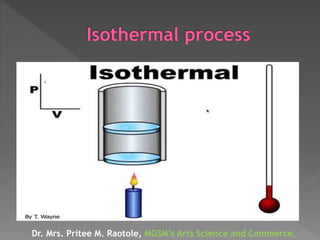
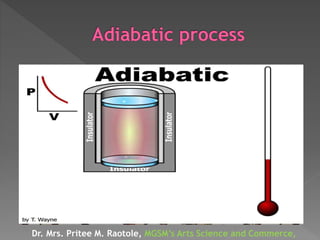
The document explains thermodynamic temperature, emphasizing its definition as an absolute measure and detailing the importance of absolute zero. It elaborates on different thermodynamic processes including isothermal, adiabatic, isochoric, and isobaric processes, highlighting their characteristics and the first law of thermodynamics as it applies to each. Various calculations related to work done, heat transfer, and changes in state during these processes are also discussed.