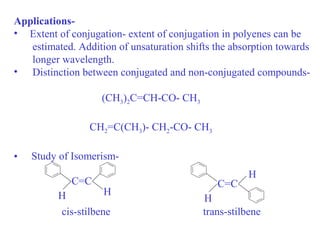
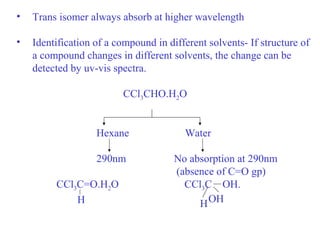
- An auxochrome is a group that does not absorb light itself but shifts the absorption bands of a chromophore to longer wavelengths in the red region of the spectrum. When combined with a chromophore, an auxochrome modifies the position of the absorption band relative to the parent chromophore. Examples include -OH, -OR, -NH2, etc.
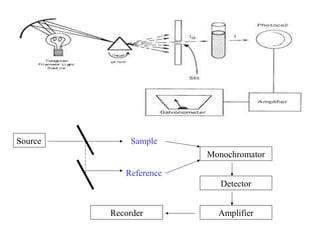
- A double beam spectrophotometer compares the intensities of light passing through a sample and reference solution. It measures absorbance and can determine concentration based on the Beer-Lambert law relationship between absorbance, concentration, and path length.
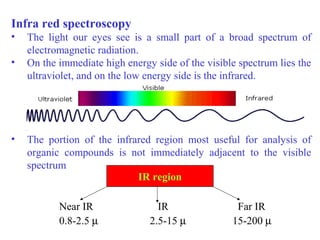
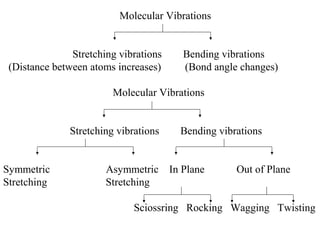
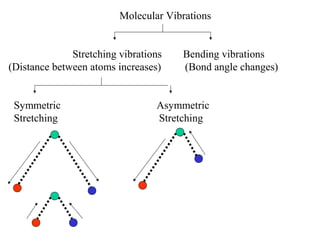
- Infrared spectroscopy analyzes the vibrational and rotational transitions of covalent bonds that correspond to characteristic absorption frequencies