Embed presentation
Download to read offline

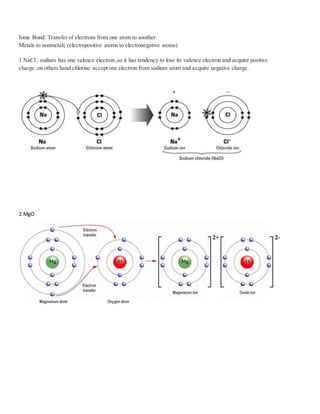
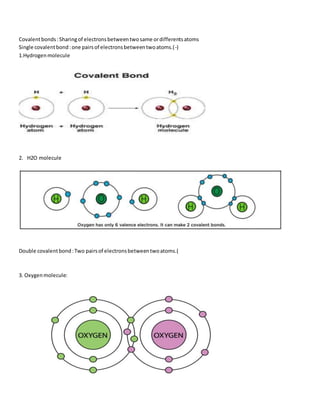
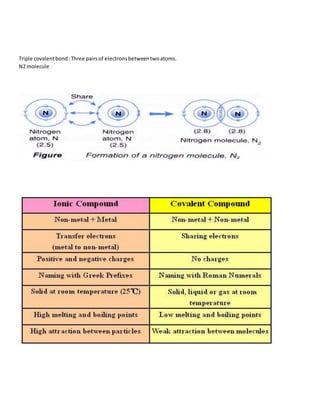
Atoms form stable chemical bonds by gaining, losing, or sharing valence electrons to achieve a stable electronic configuration of 8 electrons in their outer shell. There are four main types of chemical bonds: ionic bonds formed between metals and nonmetals by electron transfer, covalent bonds formed by electron sharing between two atoms, coordinate bonds, and metallic bonds. Ionic bonds involve transfer of electrons from electropositive to electronegative atoms, covalent bonds can be single, double, or triple depending on the number of electron pairs shared.



