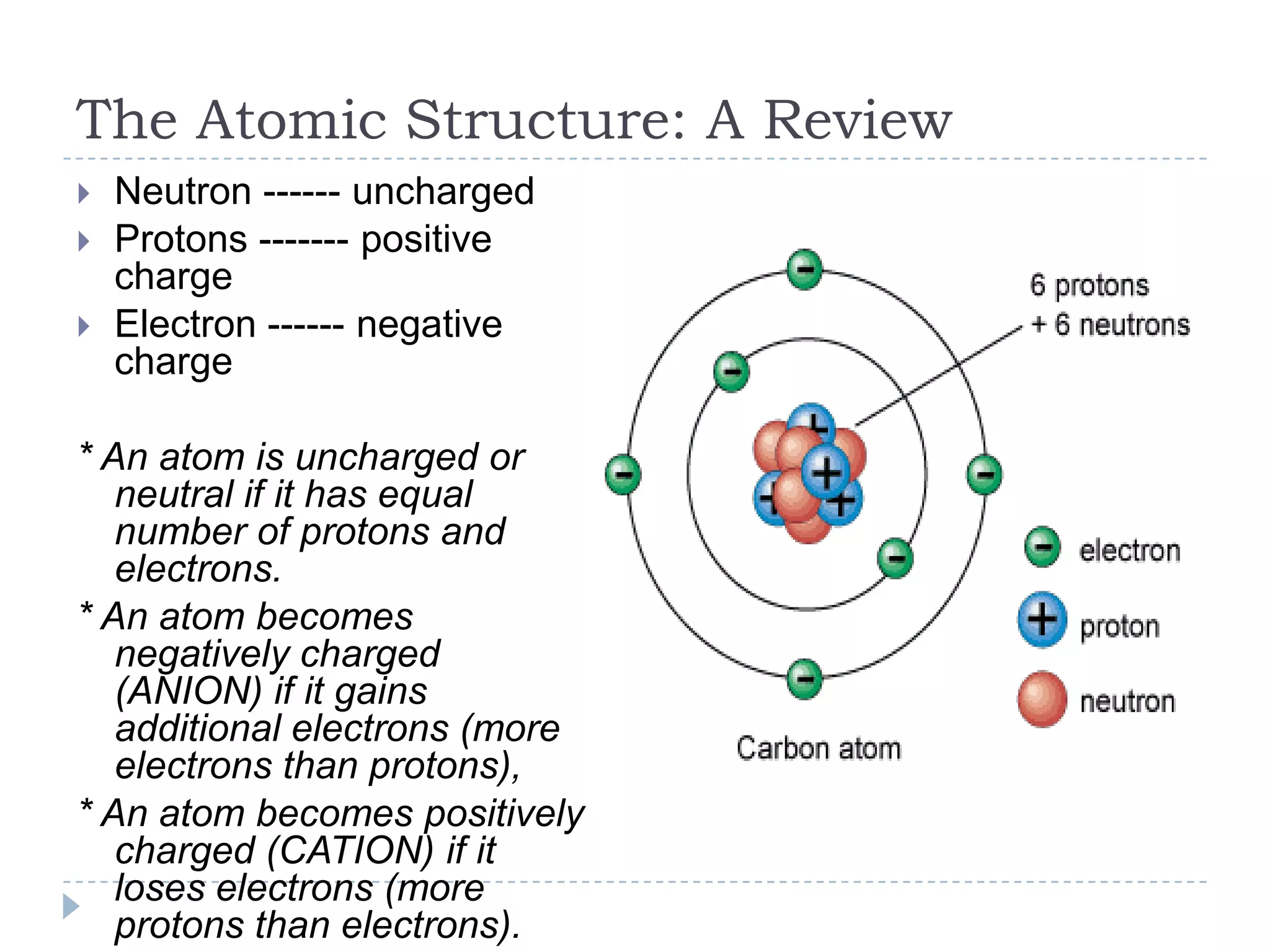
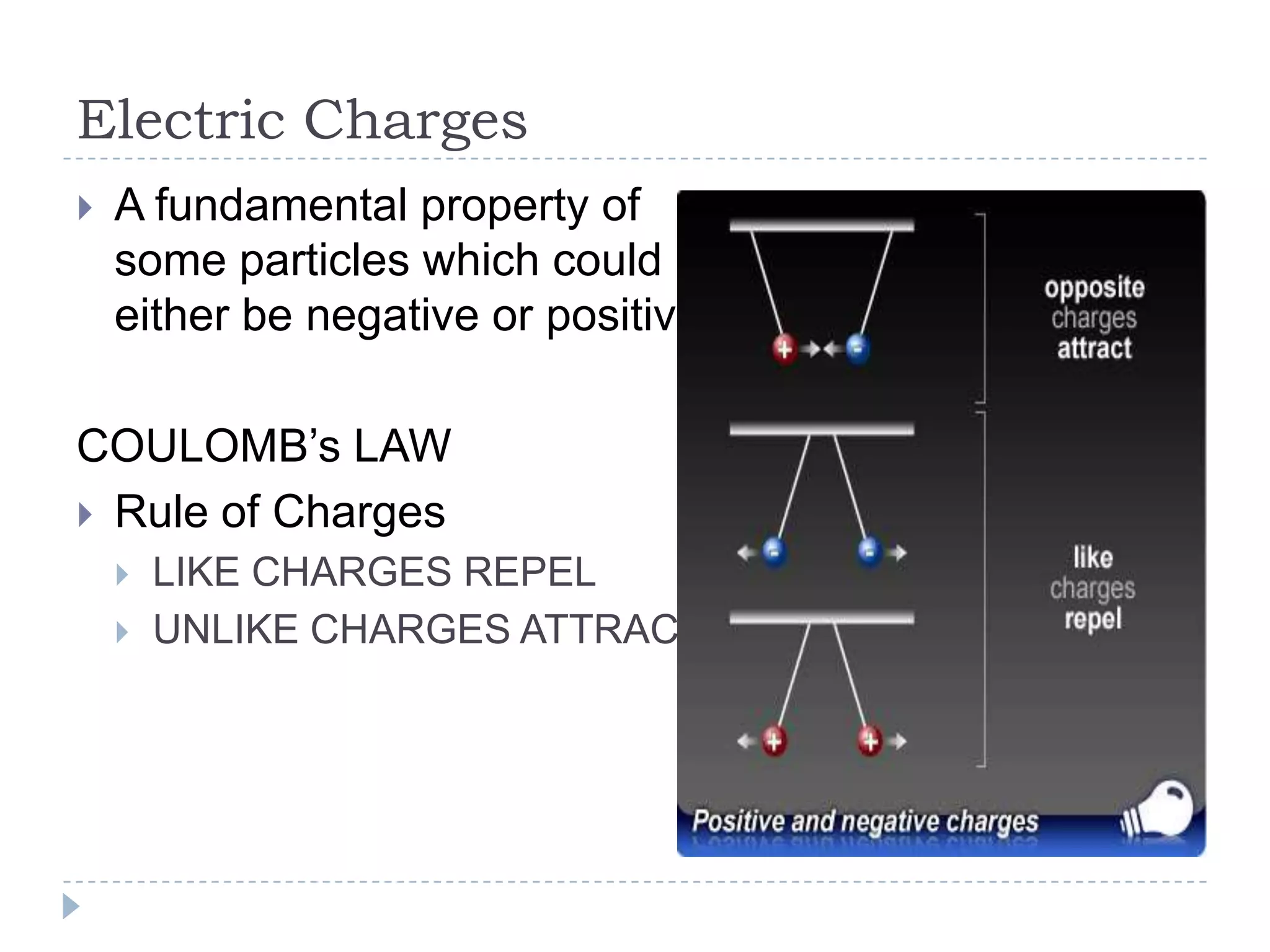
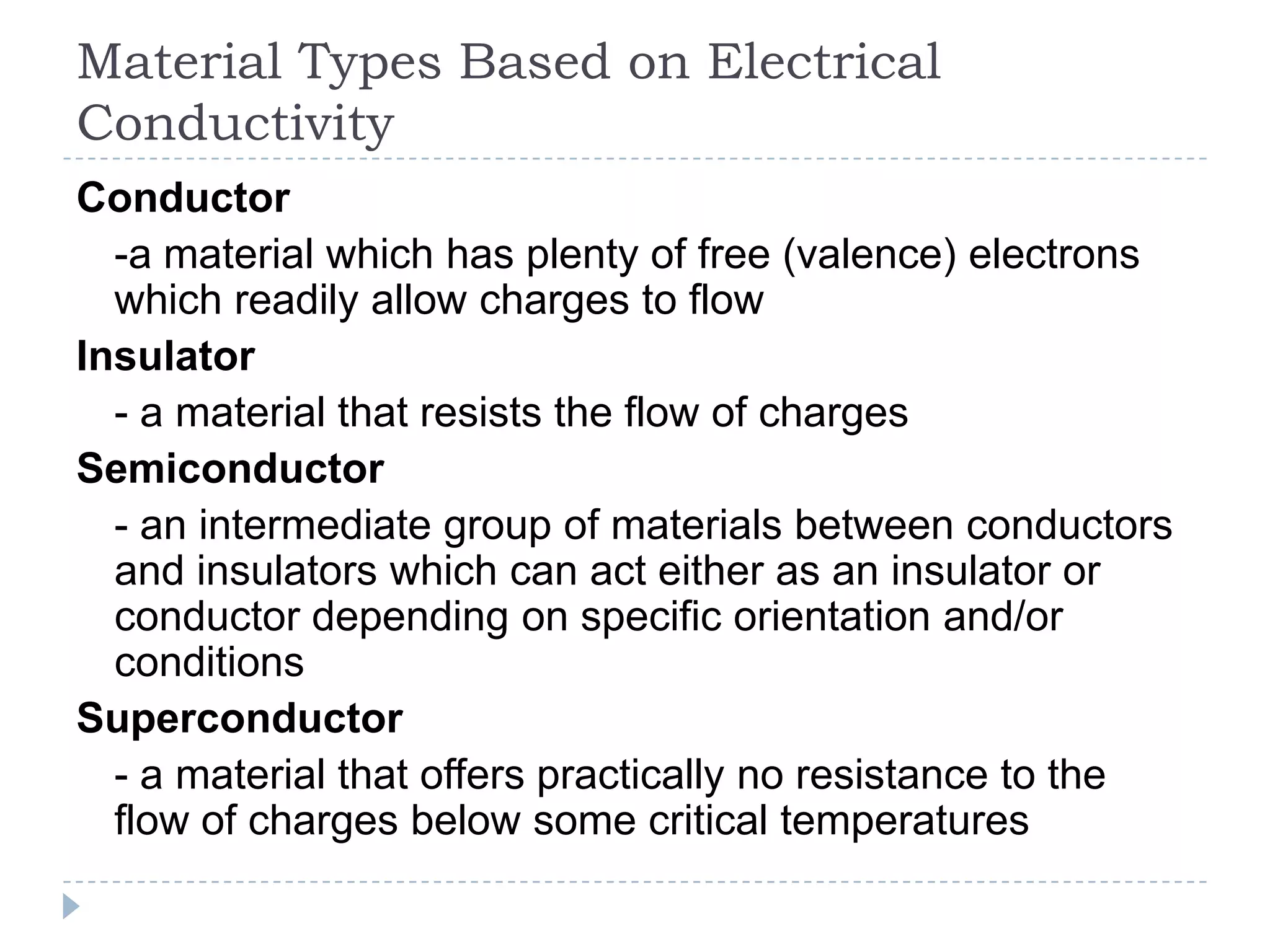
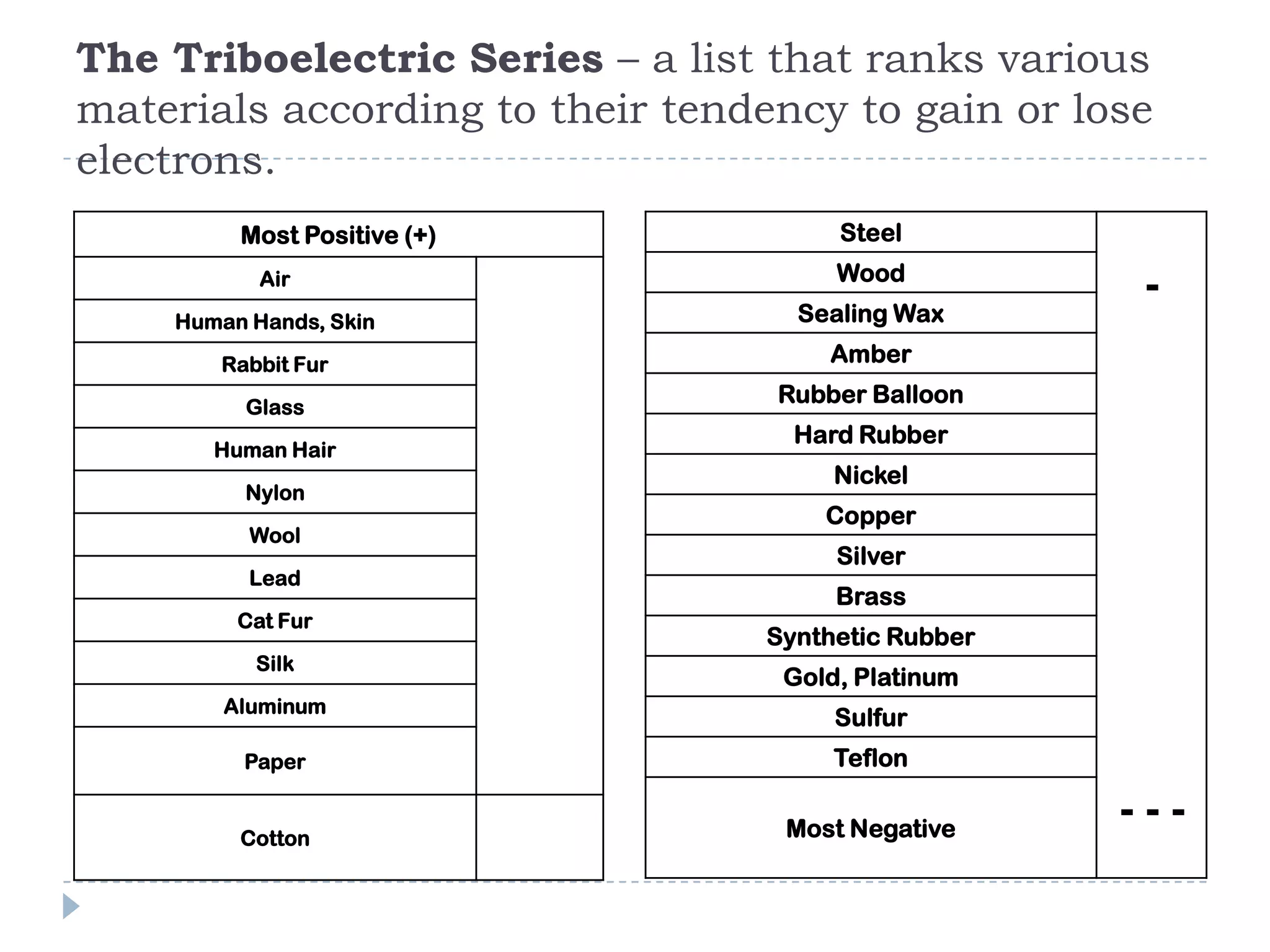
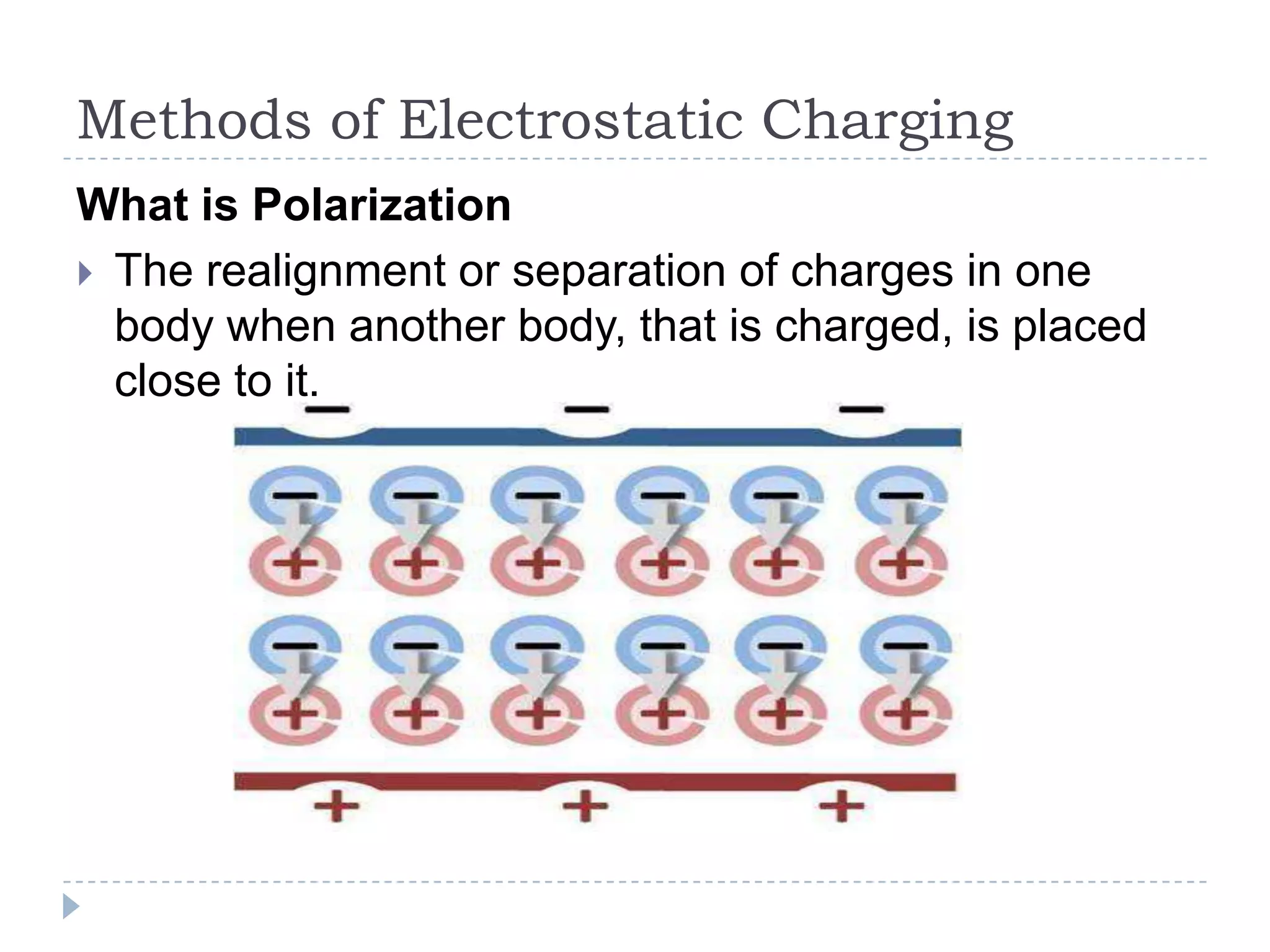
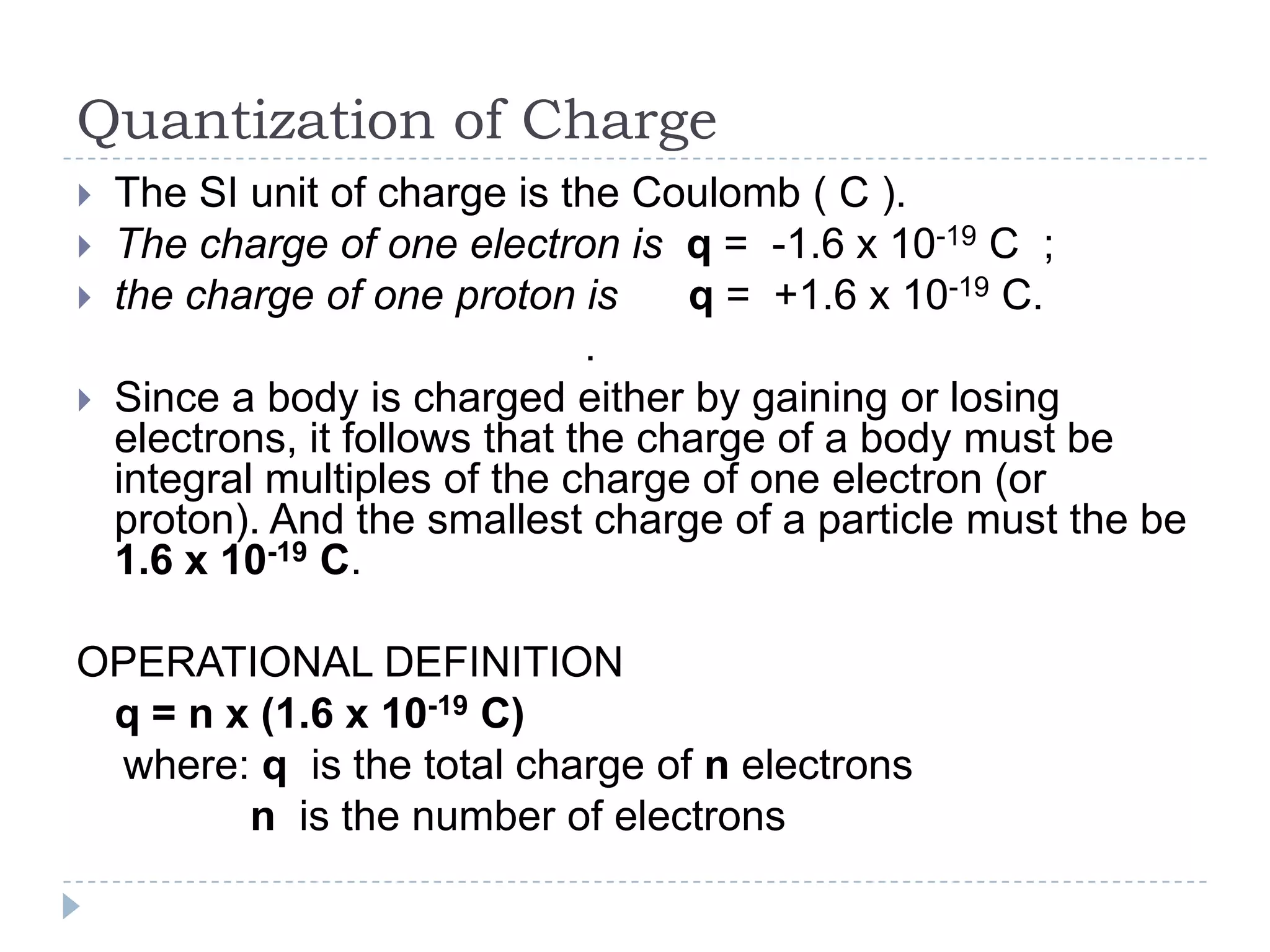
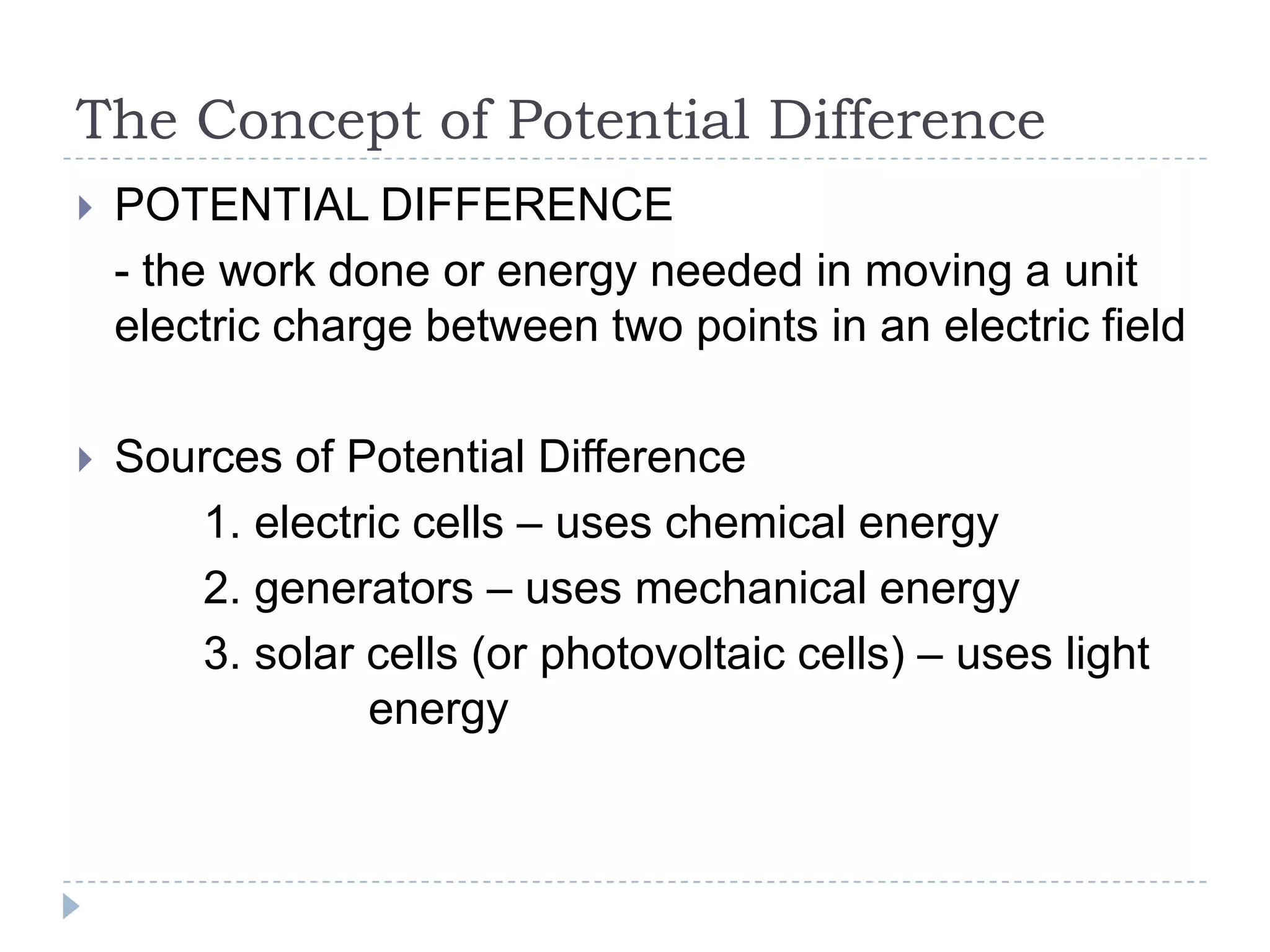
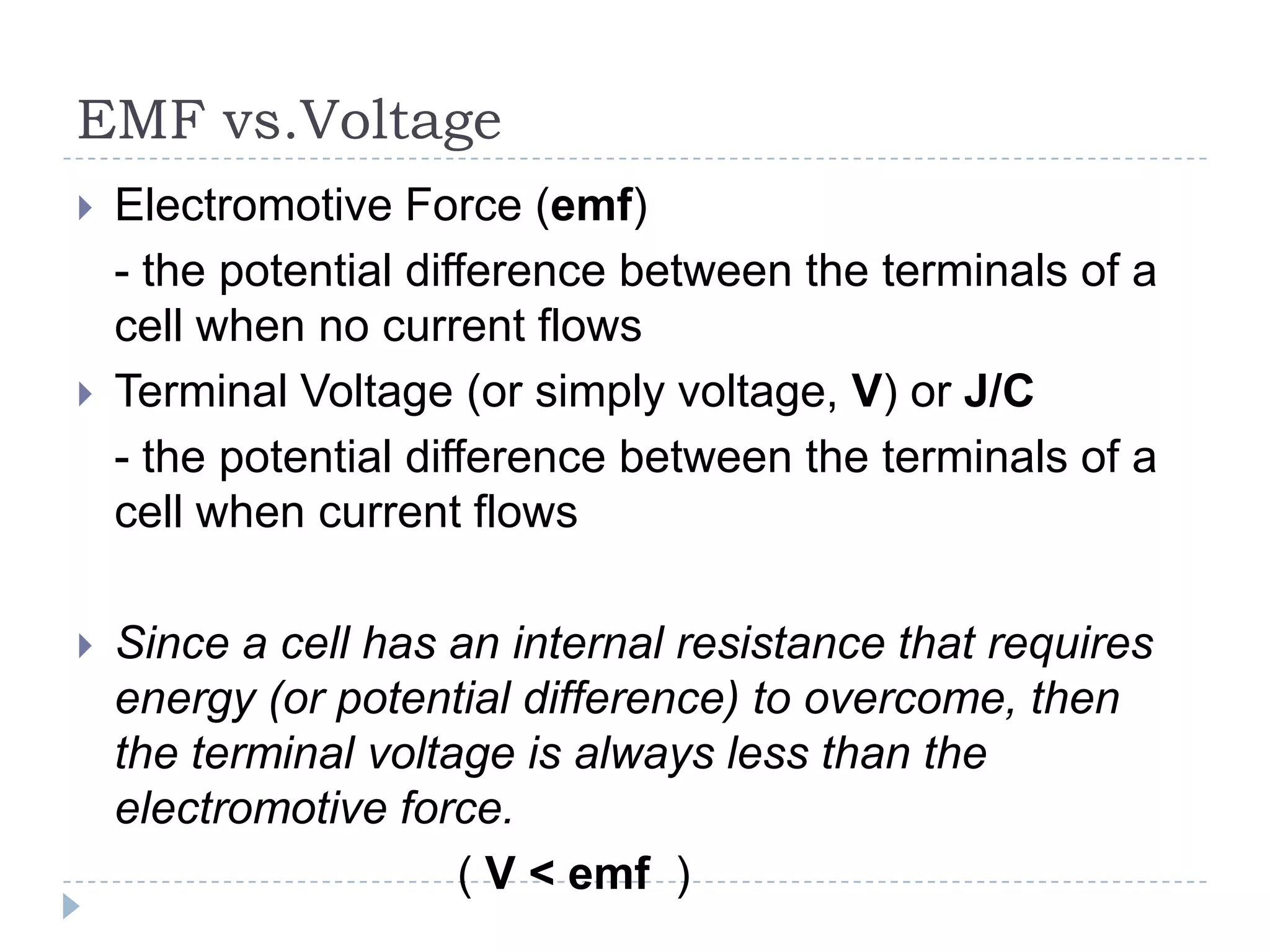
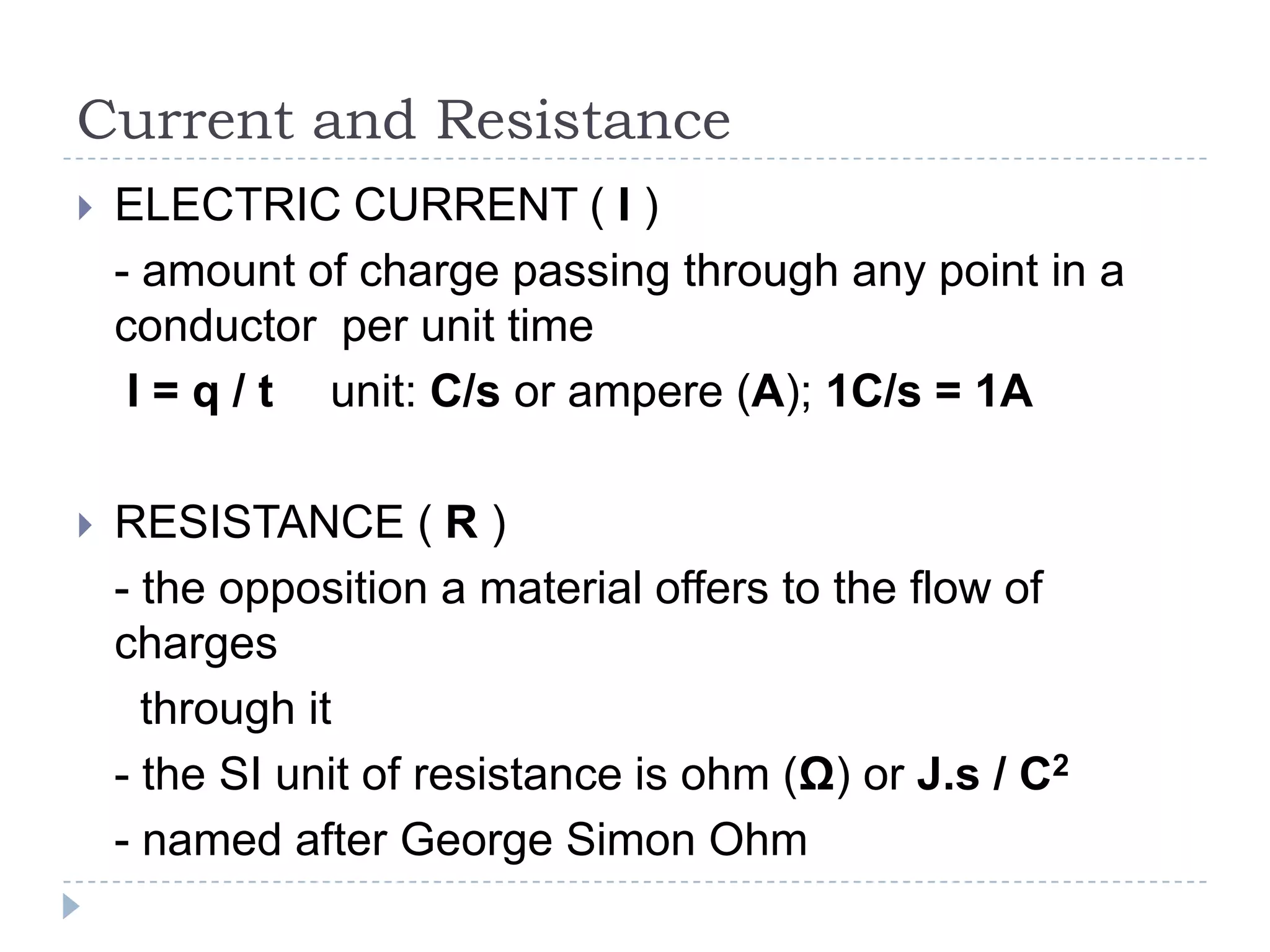
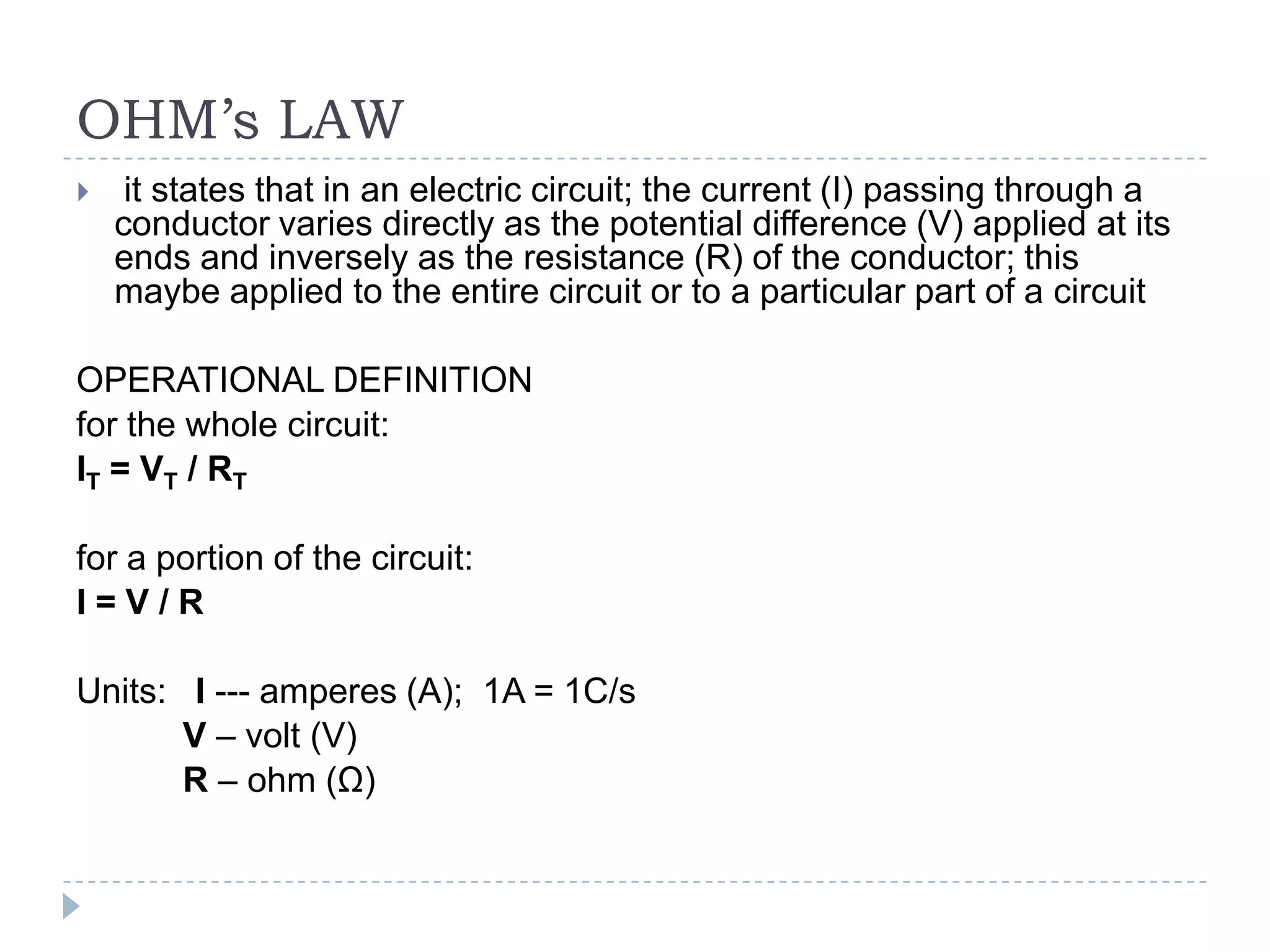
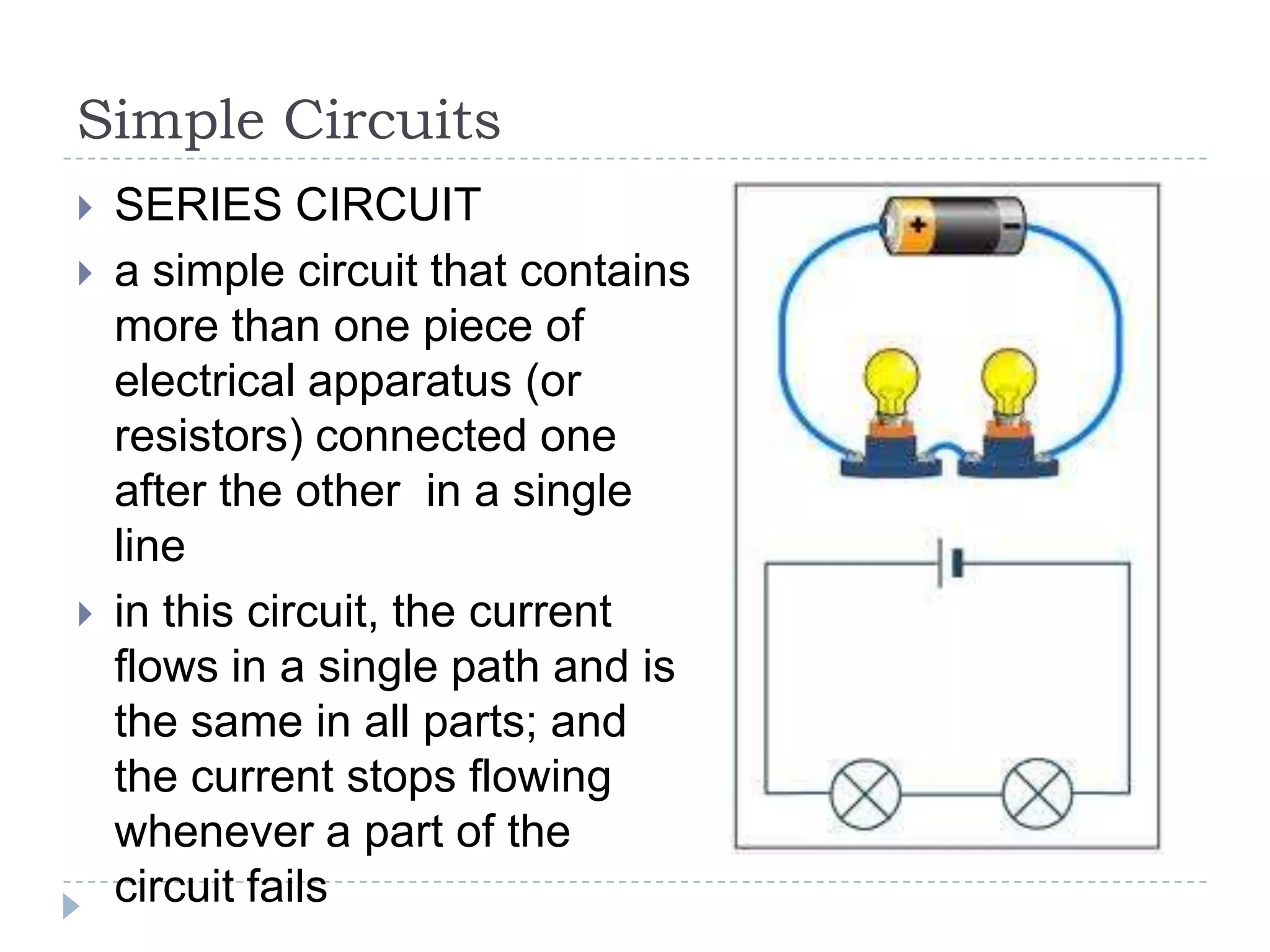
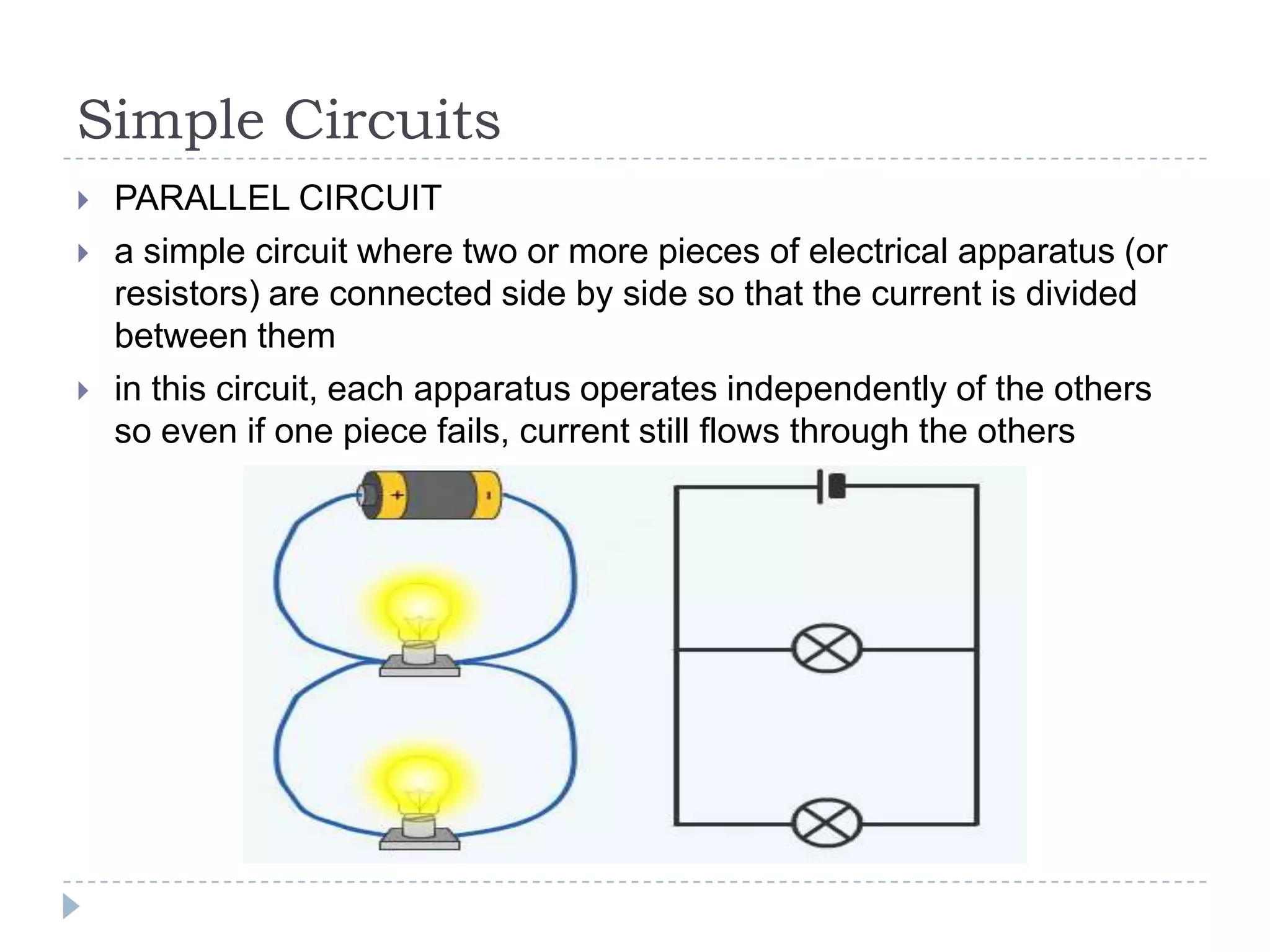
The document provides an introduction to electrostatics. It discusses the origin of electricity from observations of amber and lodestone. It describes different theories on electric charges and the atomic structure. Charging methods include charging by conduction through friction or contact, and charging by induction without contact. Materials can be conductors, insulators, or semiconductors depending on electron flow. The quantization of electric charge and basic circuit concepts such as potential difference, current, resistance, and Ohm's Law are also introduced.