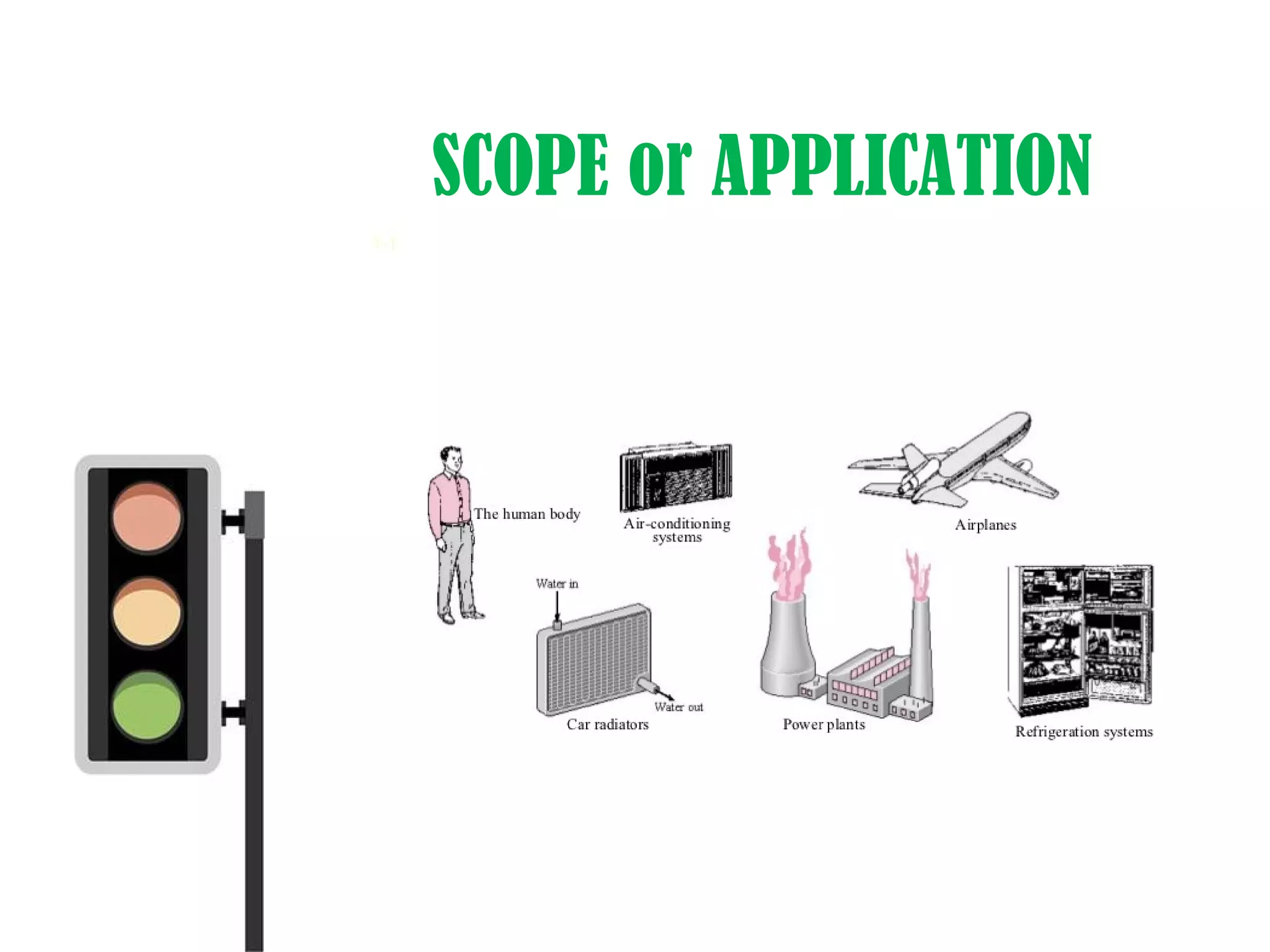
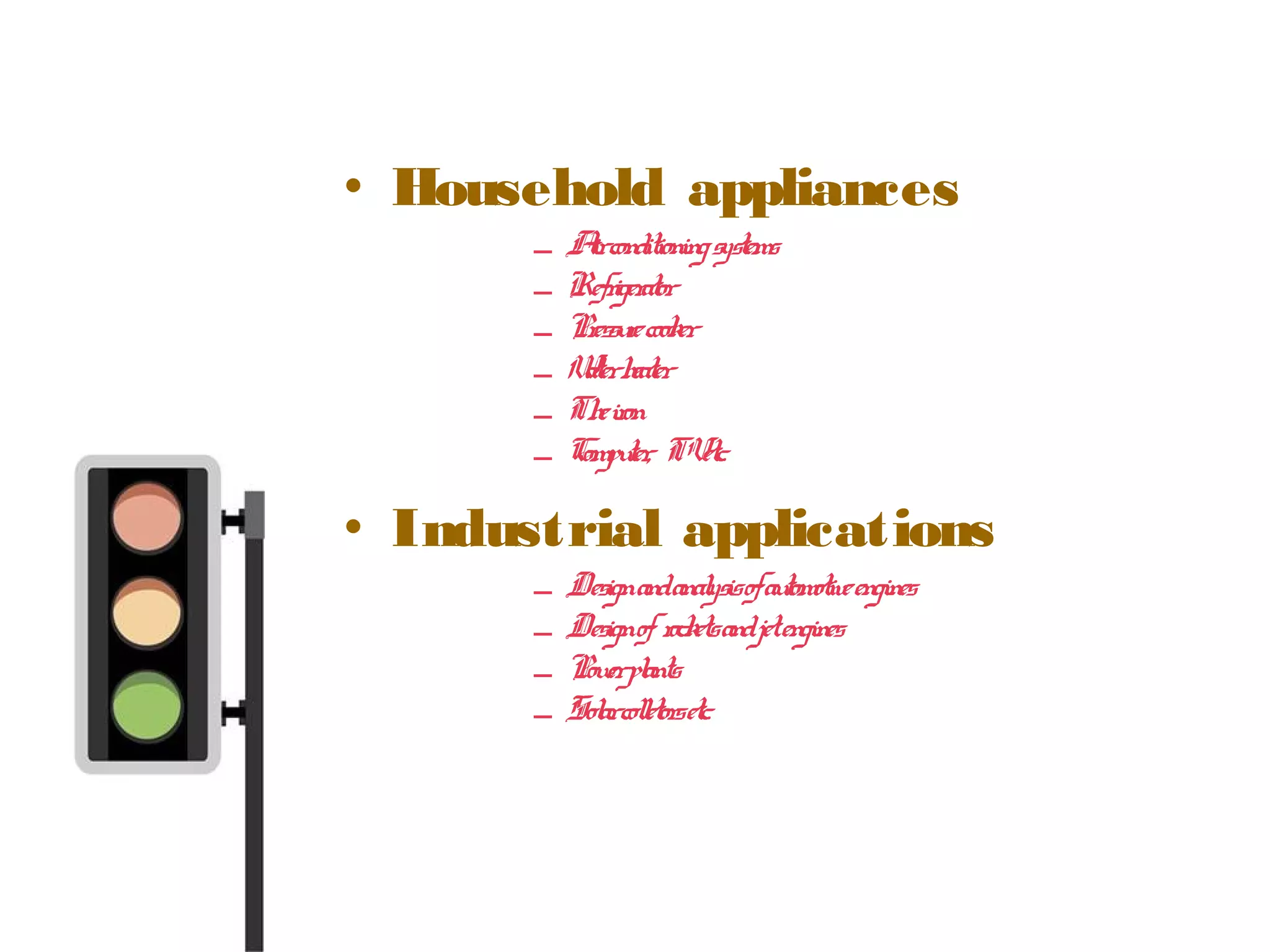
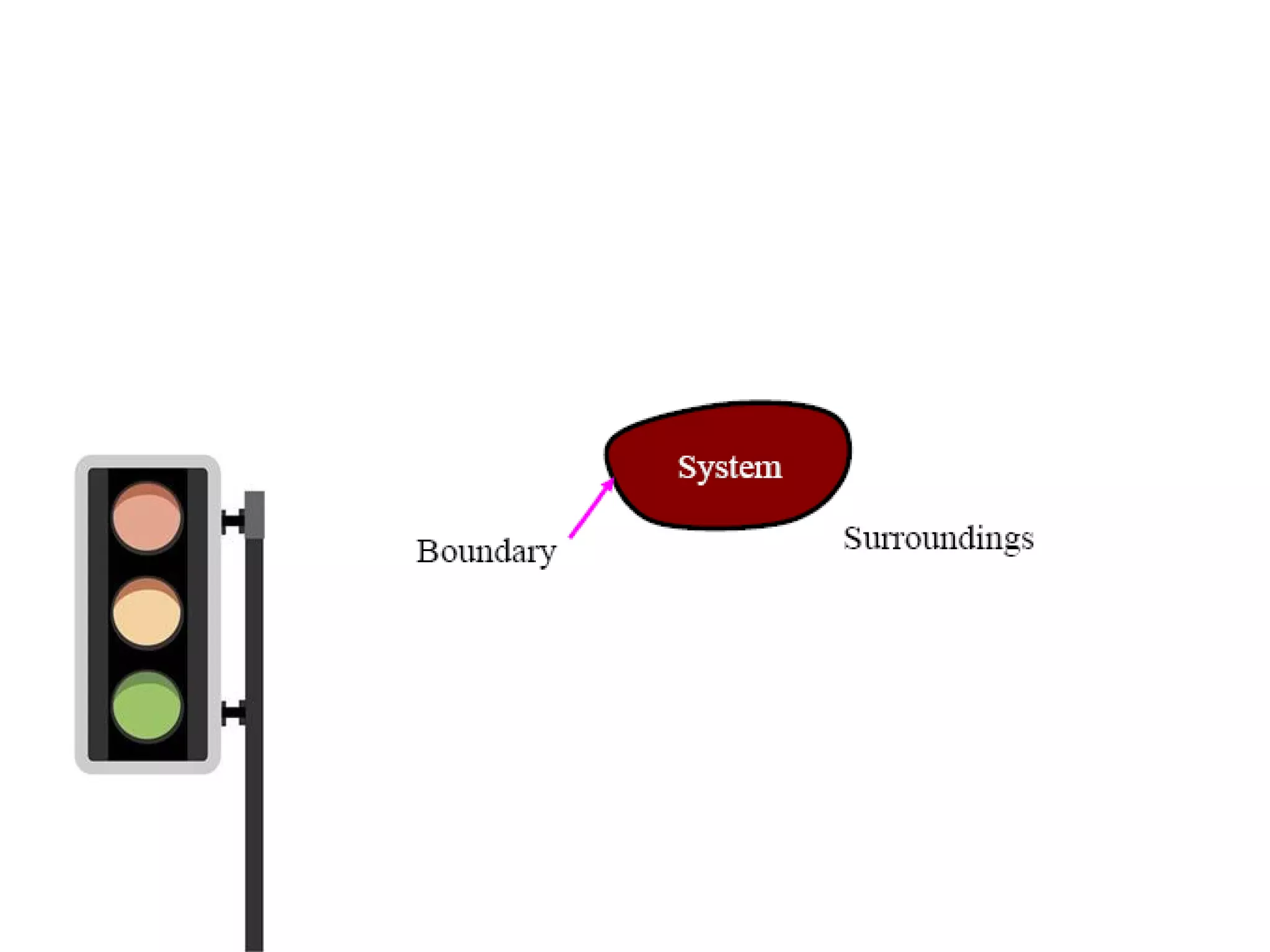
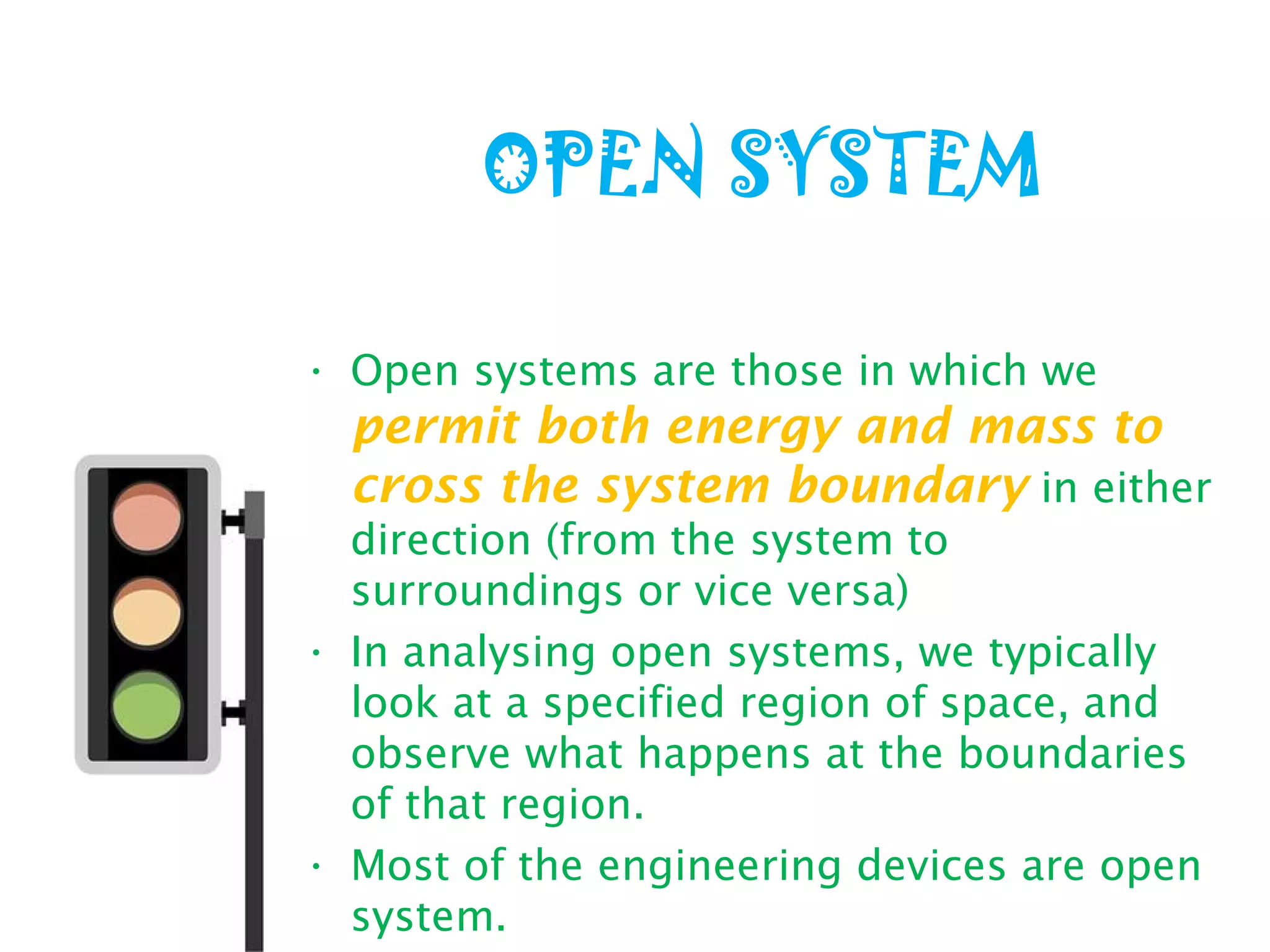
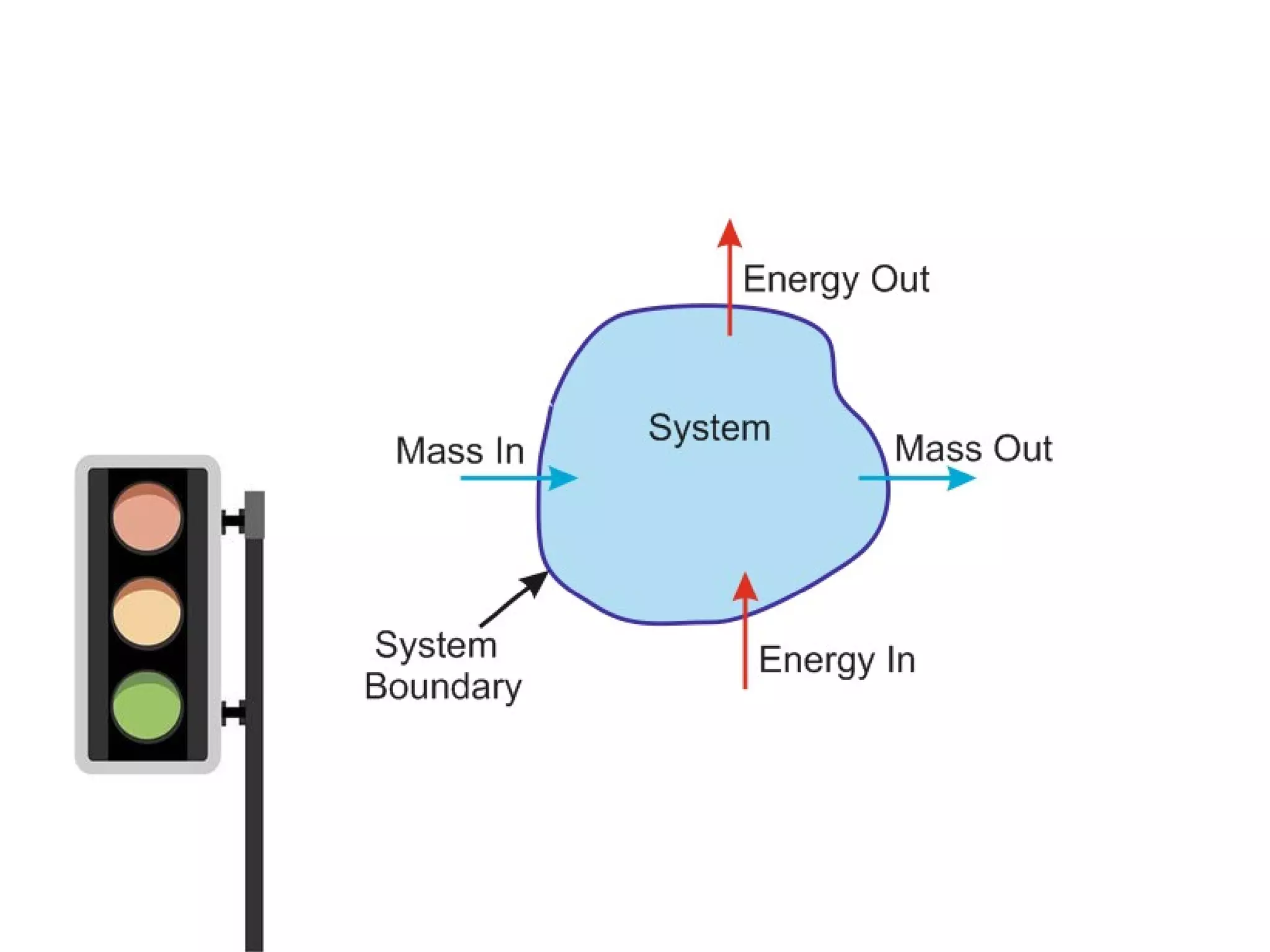
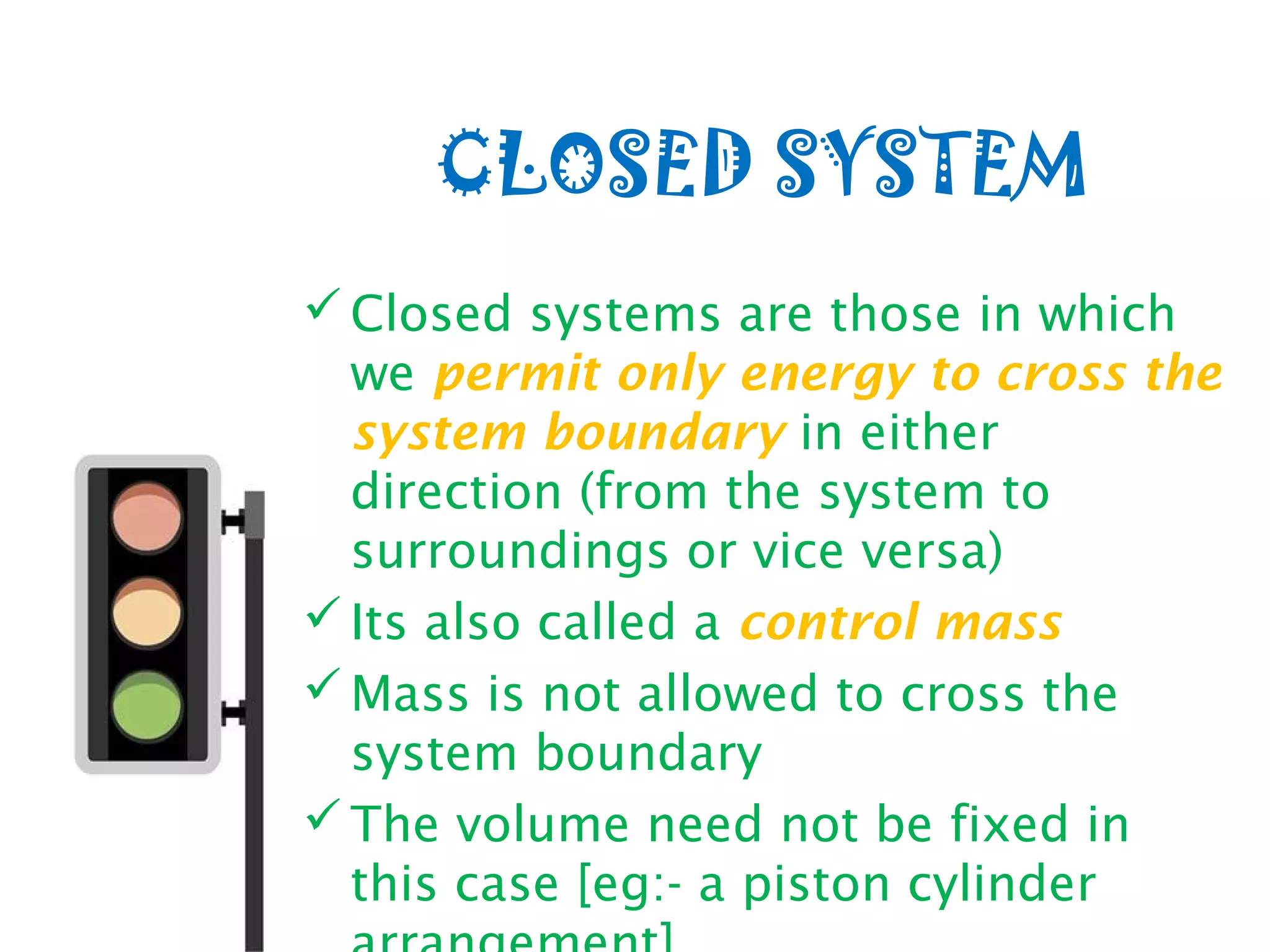
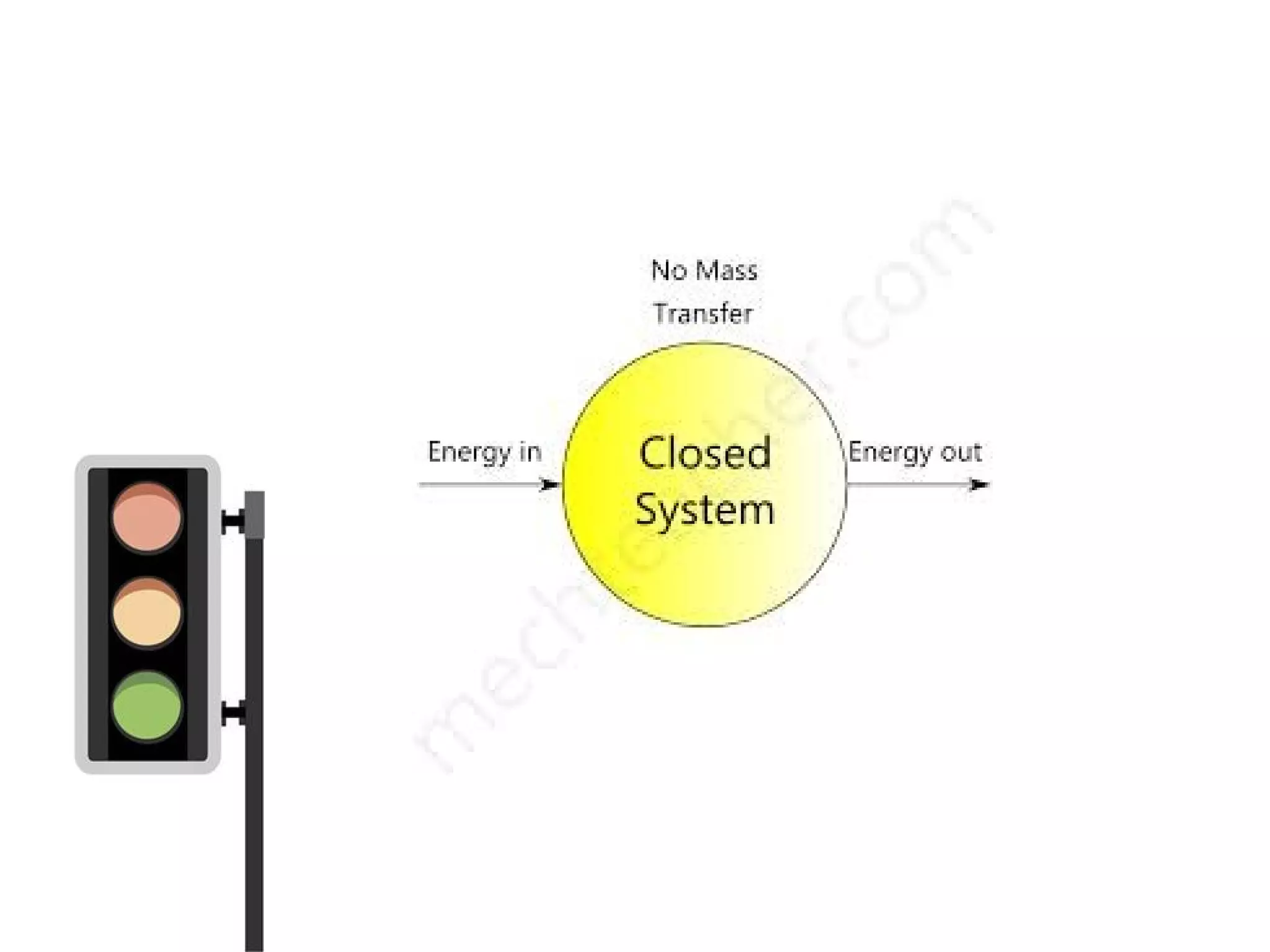
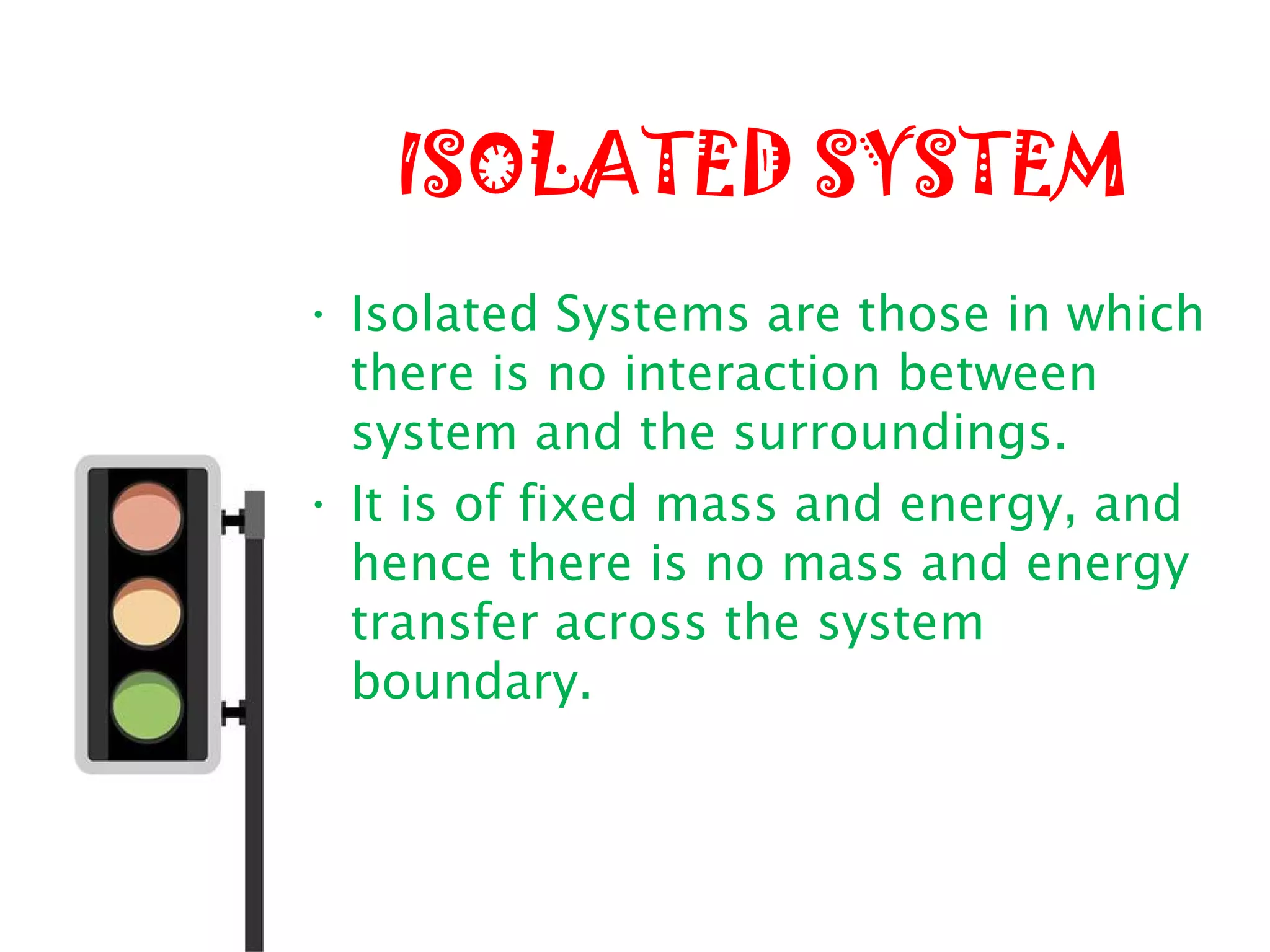
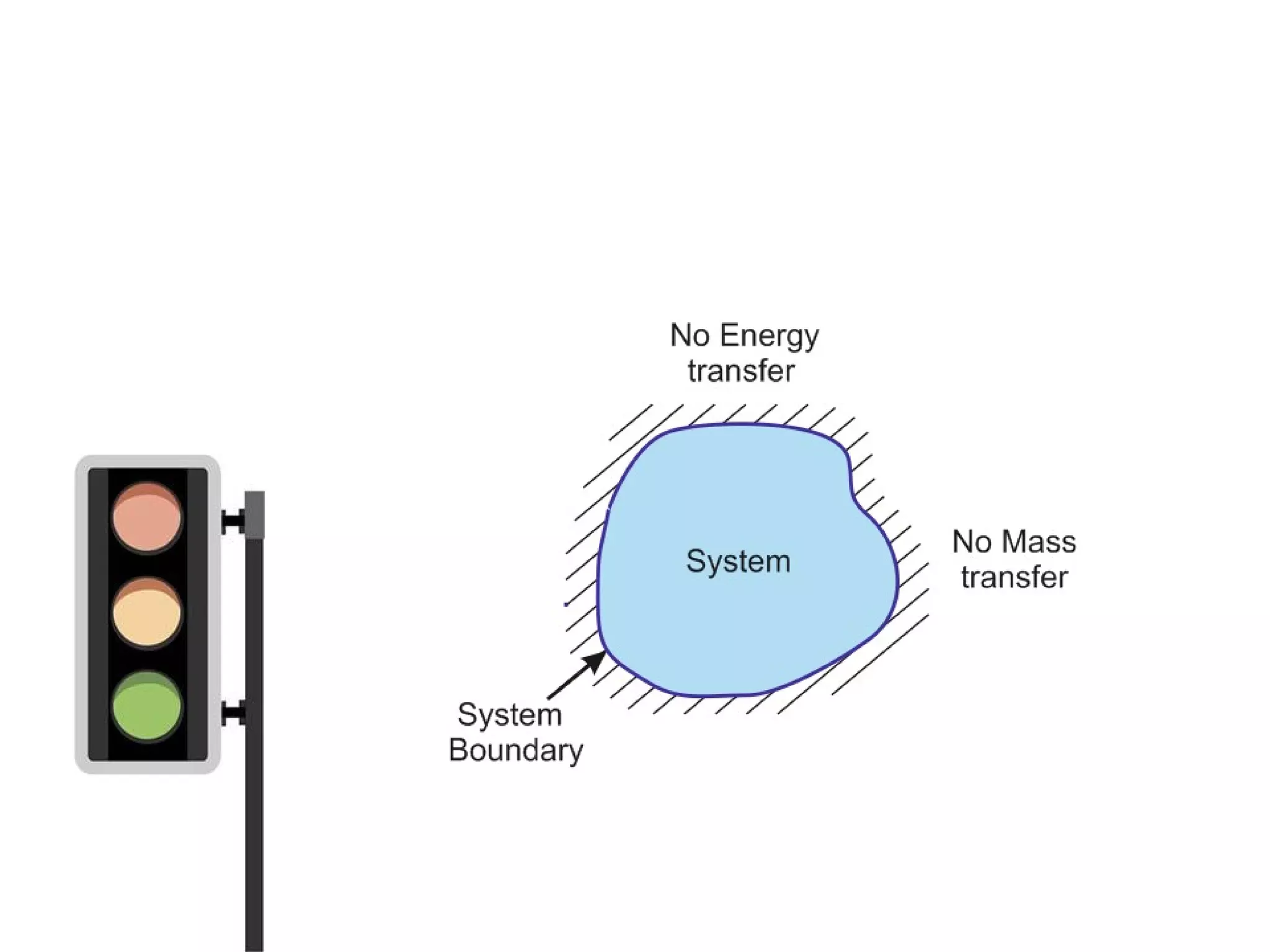
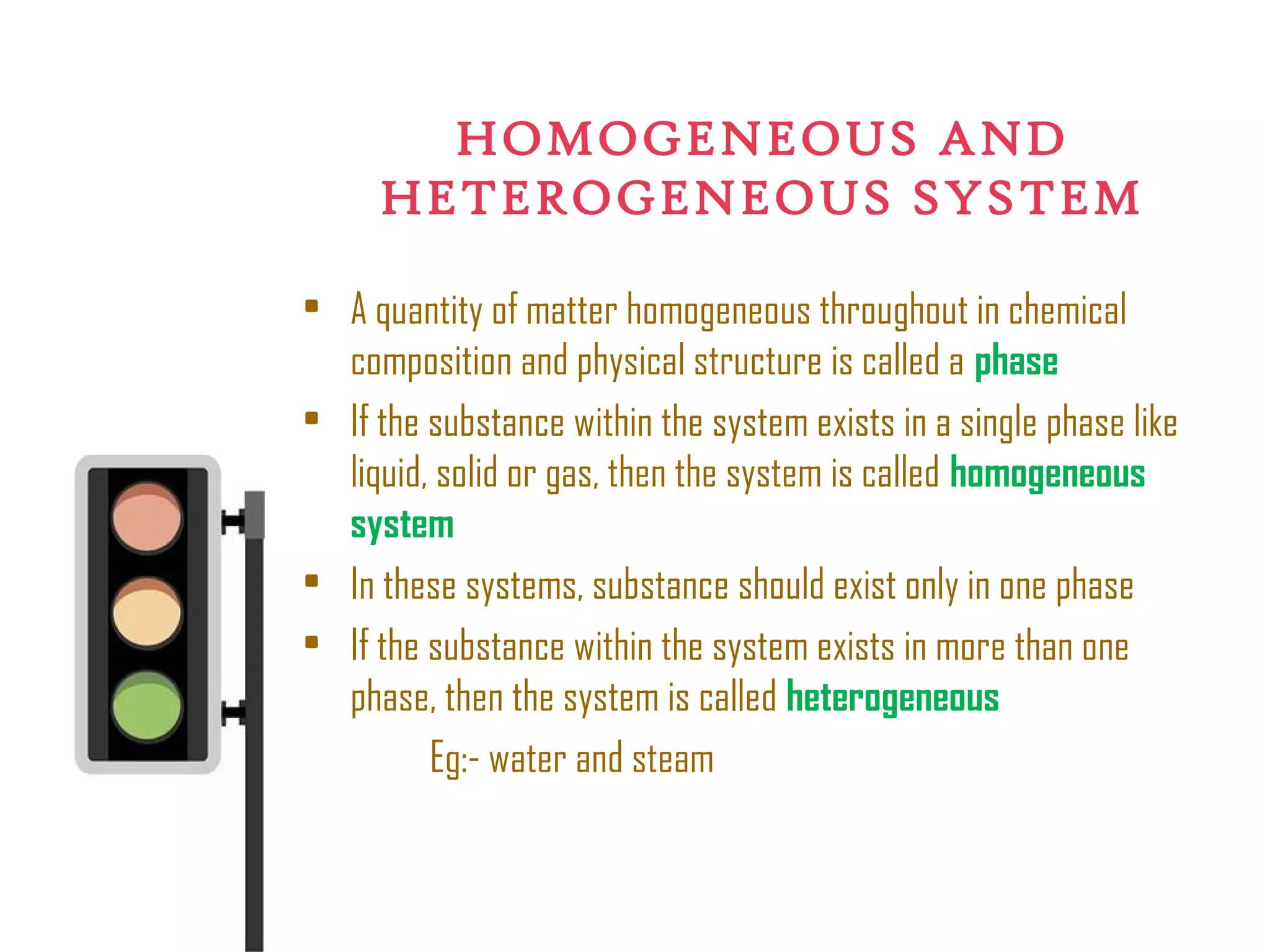
Thermodynamics is the science of energy and its transformation. It originated from the Greek words "therme" meaning heat and "dynamis" meaning power. Thermodynamics has applications in household appliances, industrial equipment like engines, and power plants. There are microscopic and macroscopic approaches to studying thermodynamics. The macroscopic approach does not require knowledge of molecular behavior while the microscopic approach considers average behavior of molecules. A system and its surroundings are separated by a boundary which can be fixed or movable. Systems can be open, closed, or isolated depending on how mass and energy cross the boundary.


![INTRODUCTION
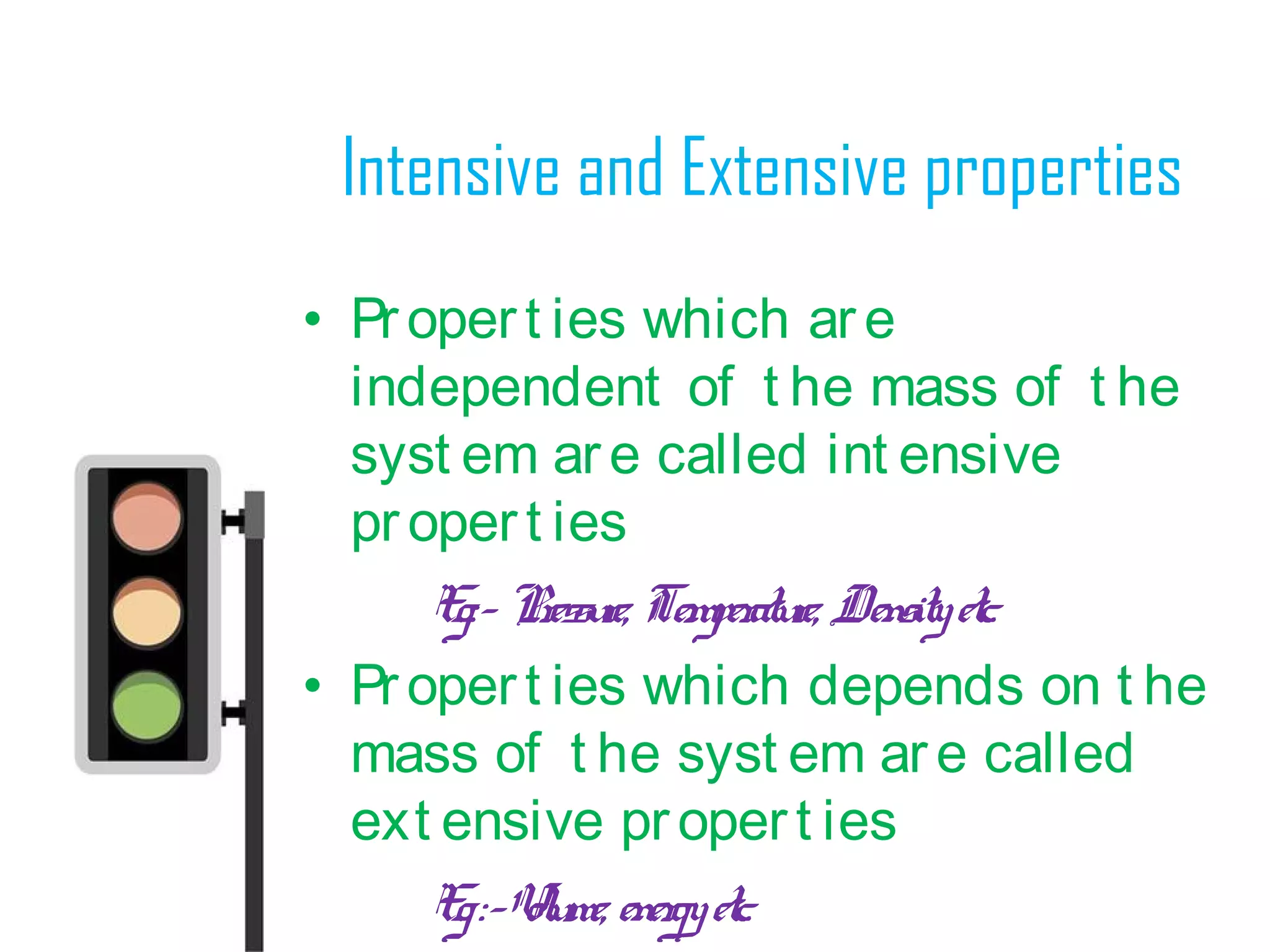
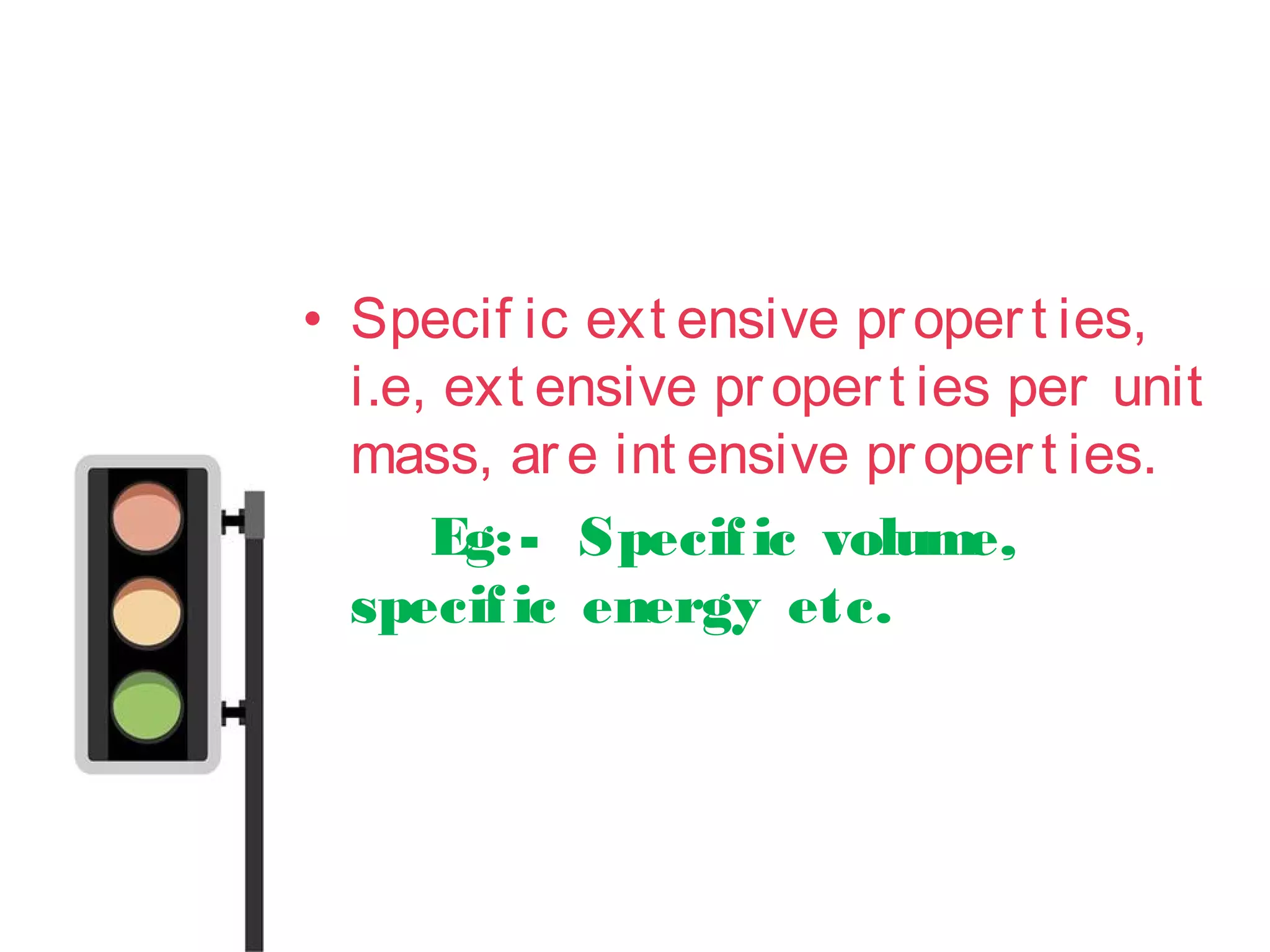
• Thermodynamics can be def ined
as t he science of energy
• Word “Thermodynamics”
originat es f rom t he greek words
t her me[heat ] and dynamis[power]
• The early underst anding of
t her modynamics cent ered around
t he concept of get t ing power
f rom hot bodies or f rom heat ,](https://image.slidesharecdn.com/tdbasics-170427080115/75/Thermodynamics-basics-3-2048.jpg)

![MICROSCOPIC APPROACH
In this method, the property
of a system is considered to
be the result of average
behaviour of large groups of
individual particles[molecules]
It is also called STATISTICAL
THERMODYNAMICS
In microscopic approach, the
effect of molecular motion is](https://image.slidesharecdn.com/tdbasics-170427080115/75/Thermodynamics-basics-8-2048.jpg)



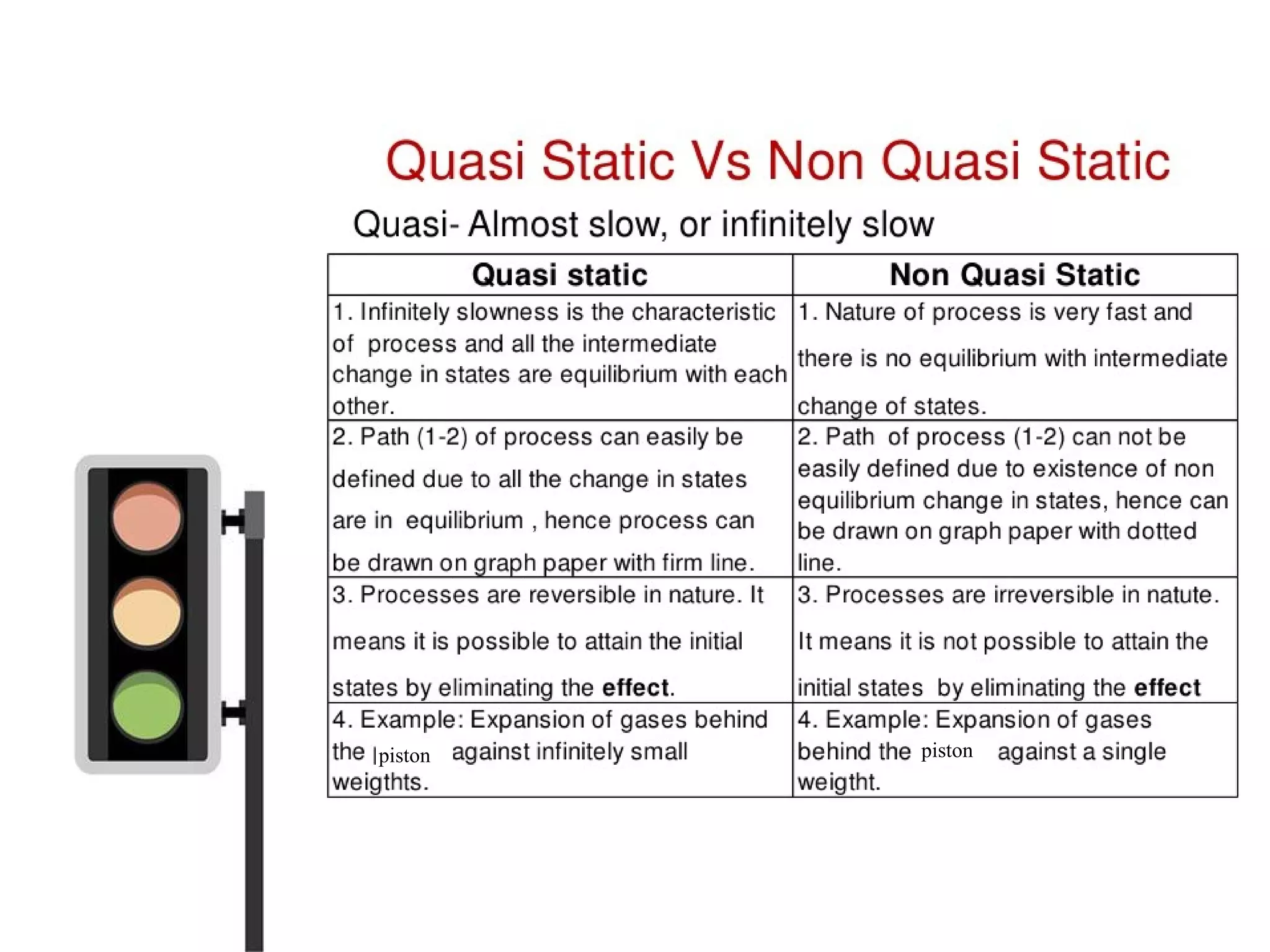

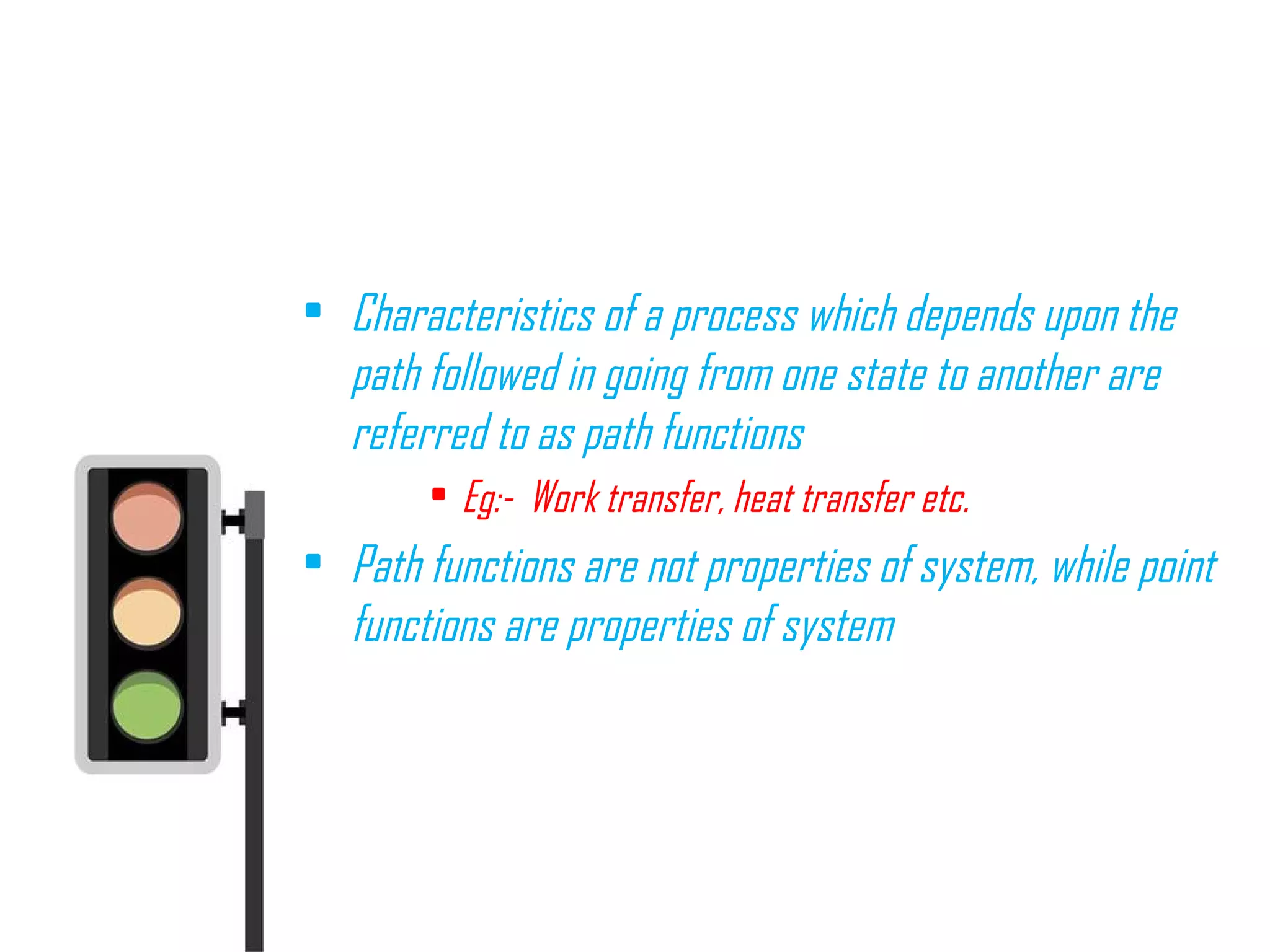
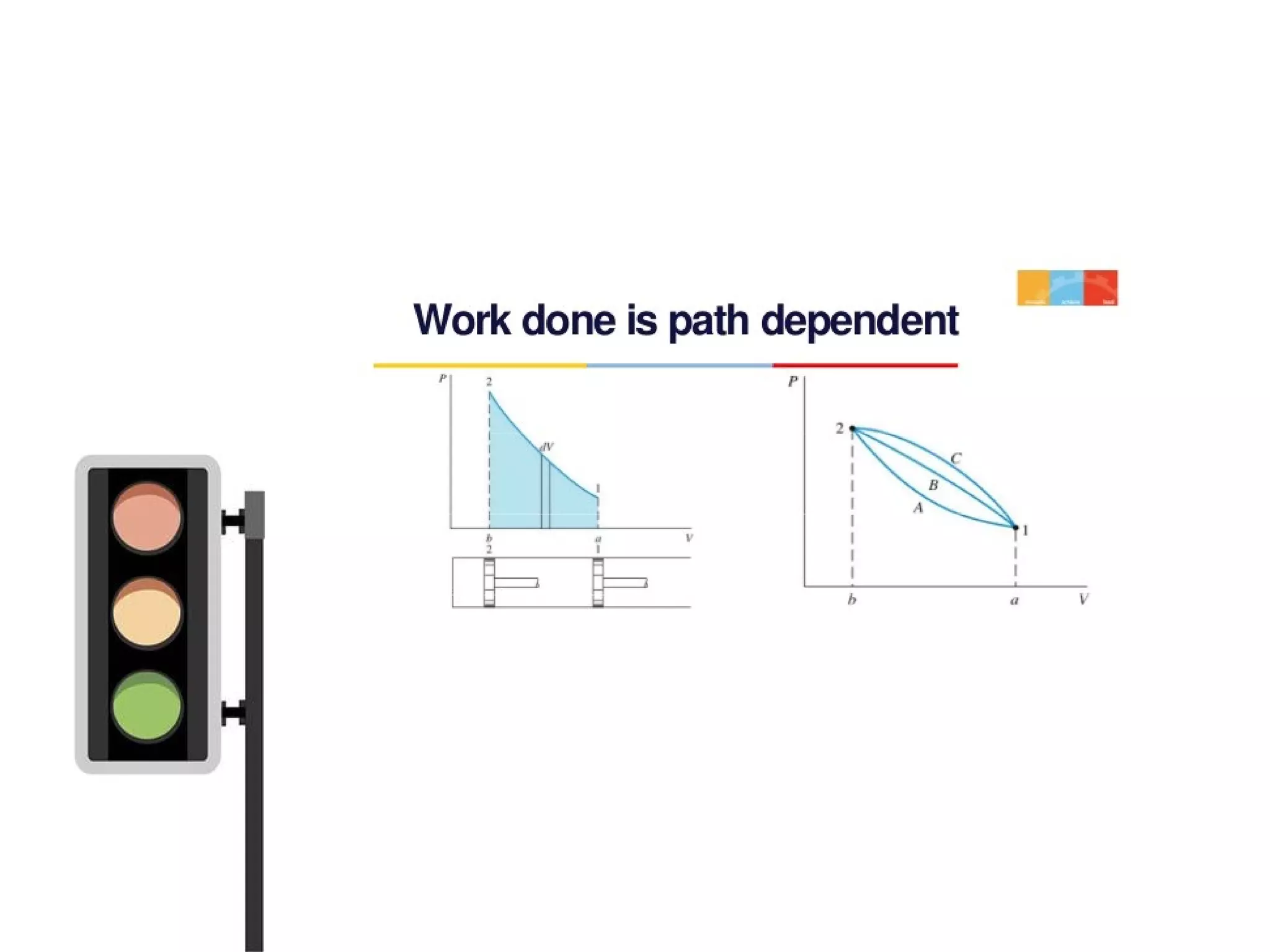
![PATH AND POINT FUNCTIONS
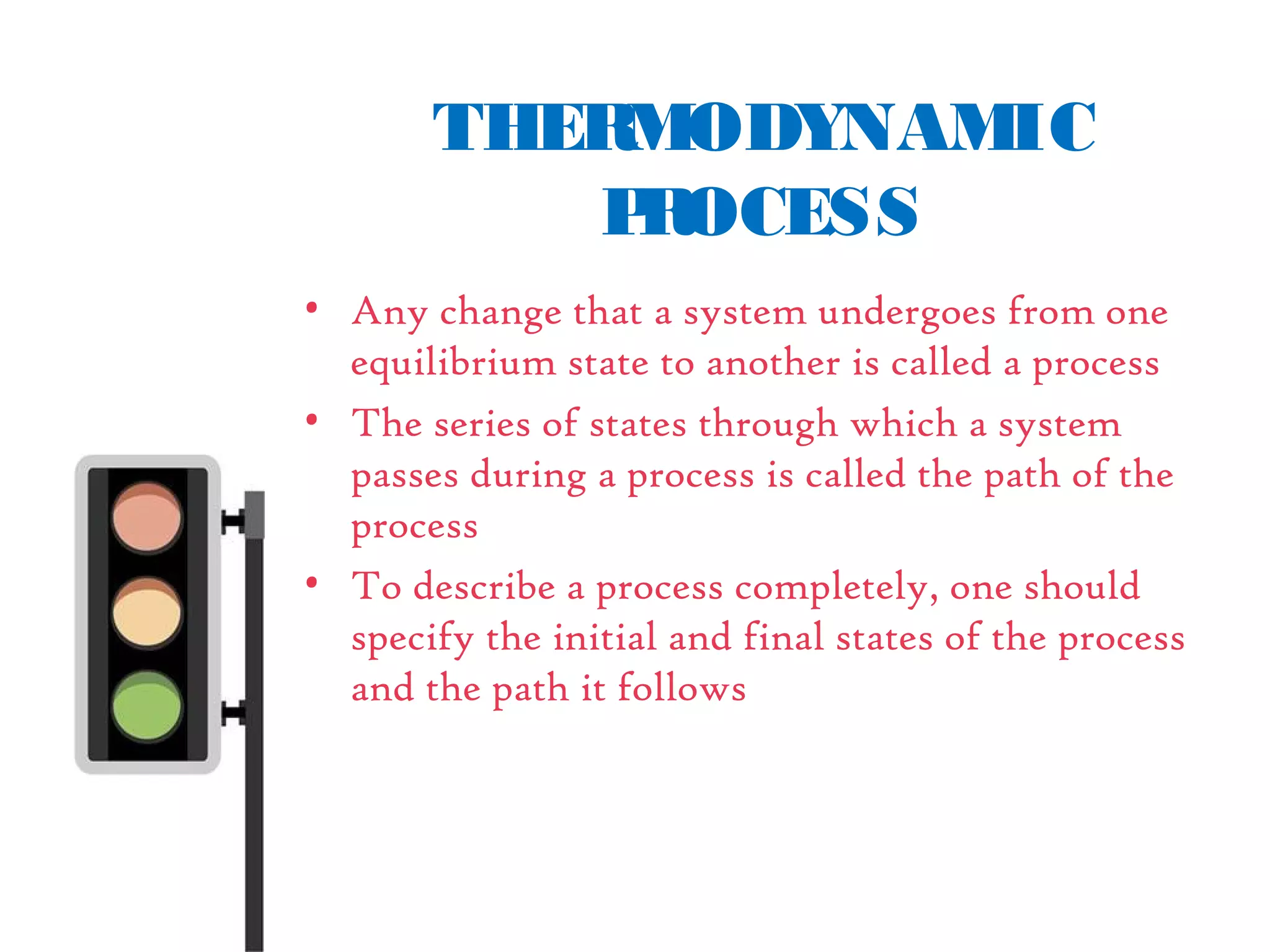
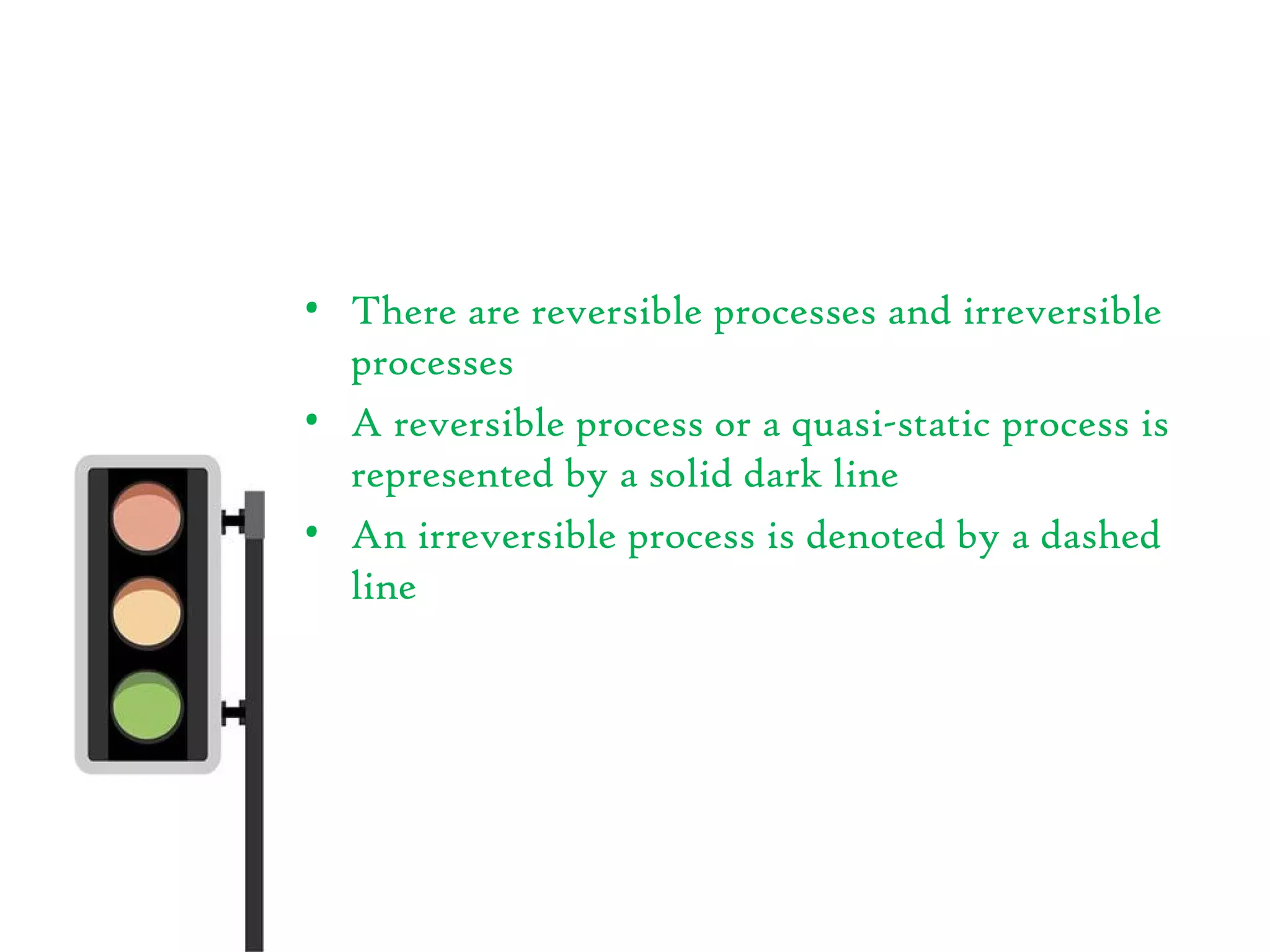
• Path isthelocusof all pointsthrough
which thesystem passesin itschange
from onestateto another
• It ispossibleto go from state1 to state
2 along different pathsasshown in the
figure[i.e, thorough A, B or C]
• Propertieslikepressure, temperature,
volumeetc. doesnot depend on the
path followed in reaching thestate, but](https://image.slidesharecdn.com/tdbasics-170427080115/75/Thermodynamics-basics-34-2048.jpg)