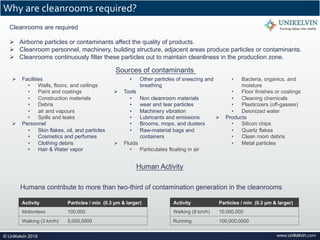
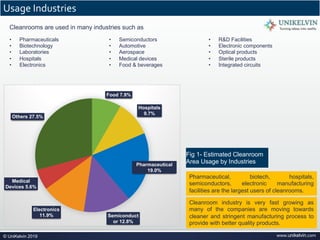
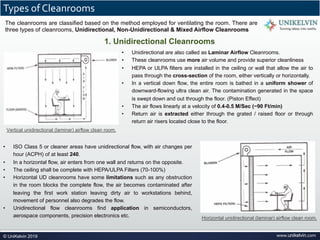
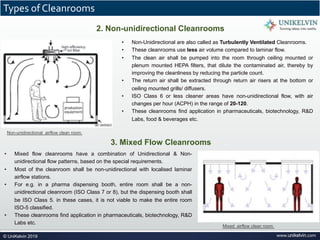
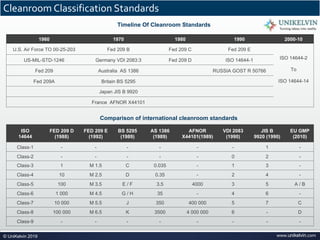
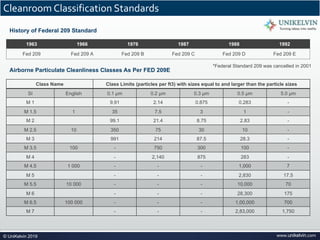
The document provides a comprehensive overview of cleanrooms, including their definition, historical development, and various types utilized across industries such as pharmaceuticals and electronics. Cleanrooms are designed to minimize airborne contaminants and are classified based on ventilation methods, adherence to international standards like ISO 14644, and their application in research and manufacturing processes. Key reasons for cleanroom requirements include maintaining product quality, mitigating contamination from personnel and materials, and ensuring a controlled environment necessary for sensitive processes.