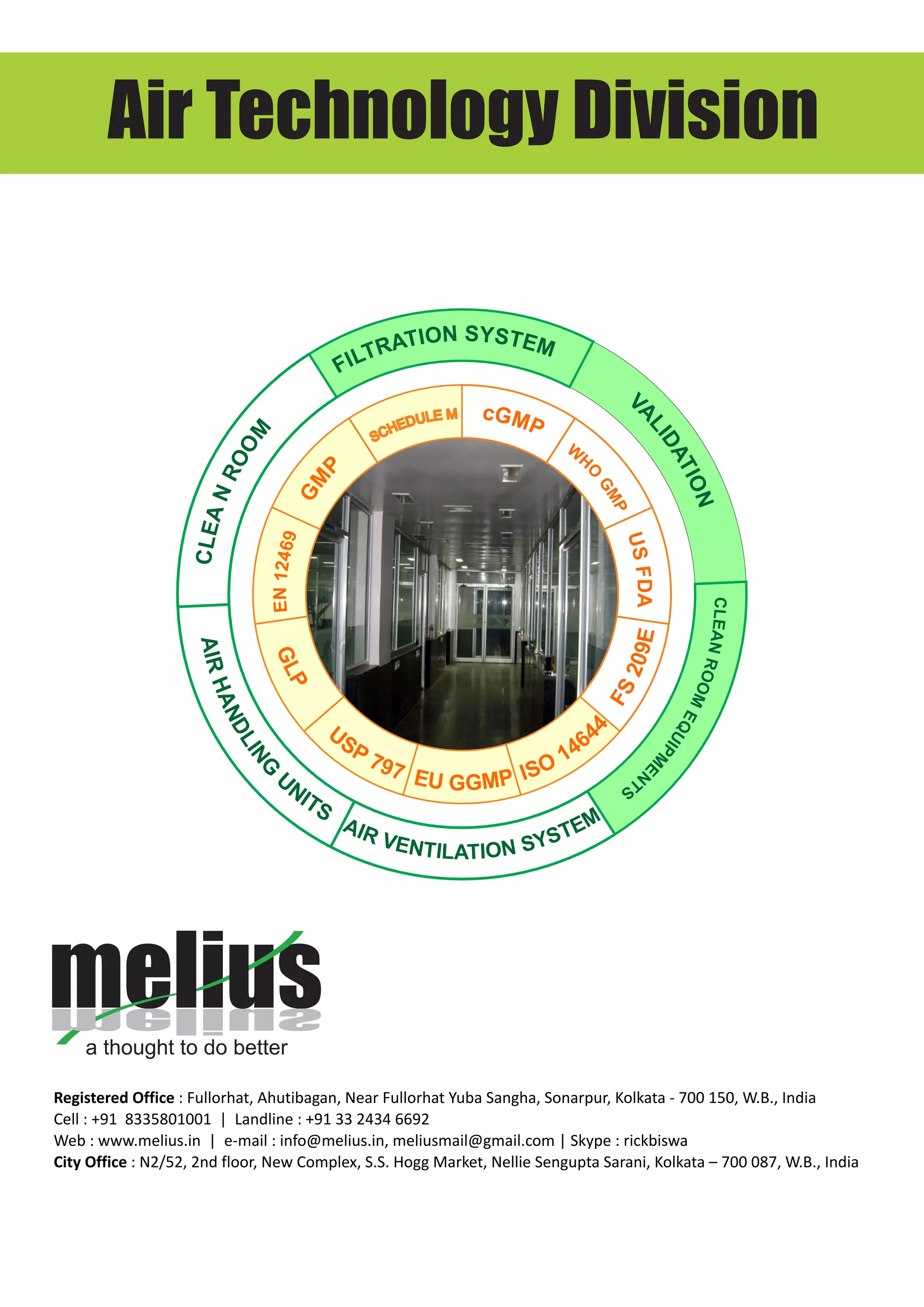
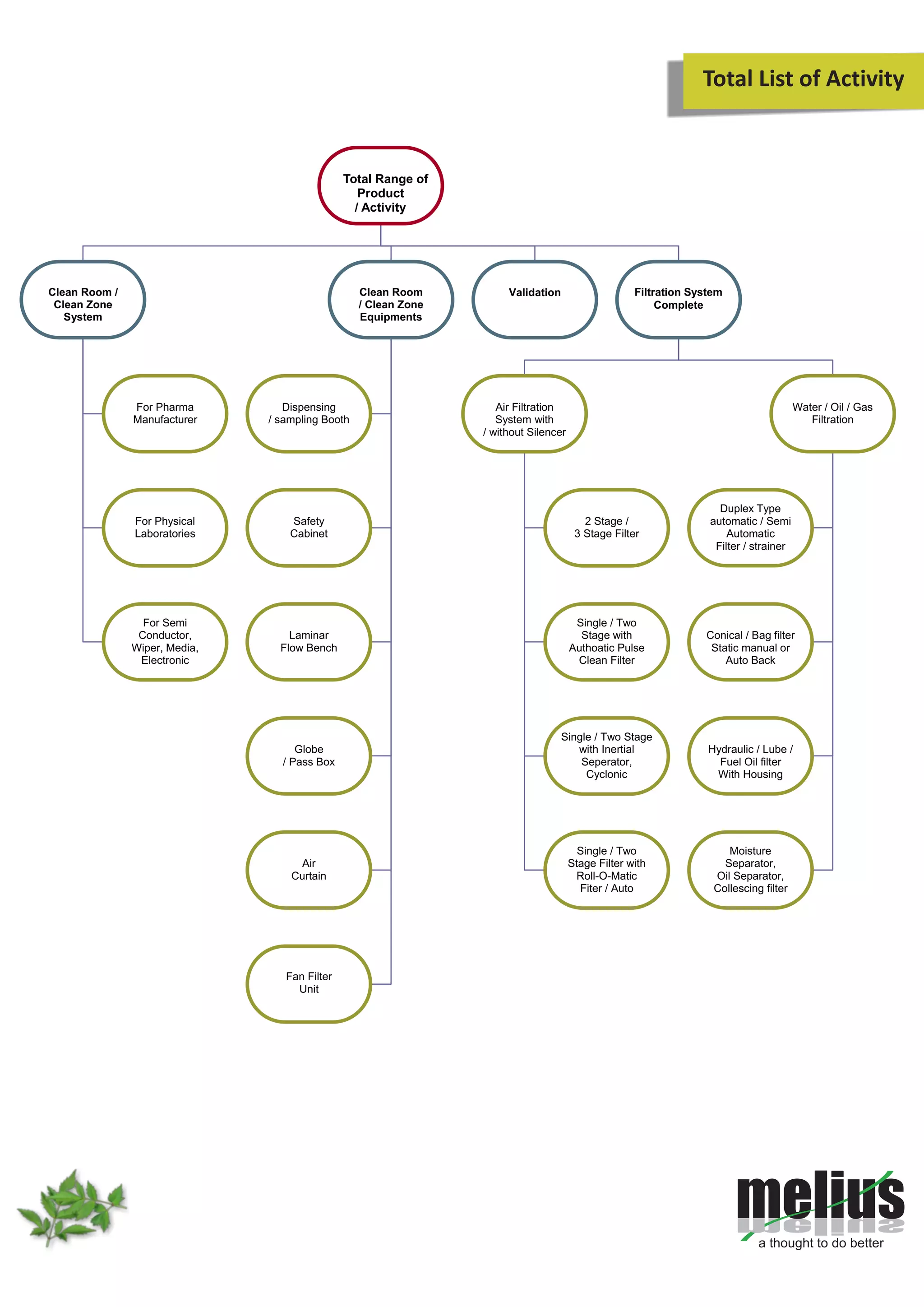
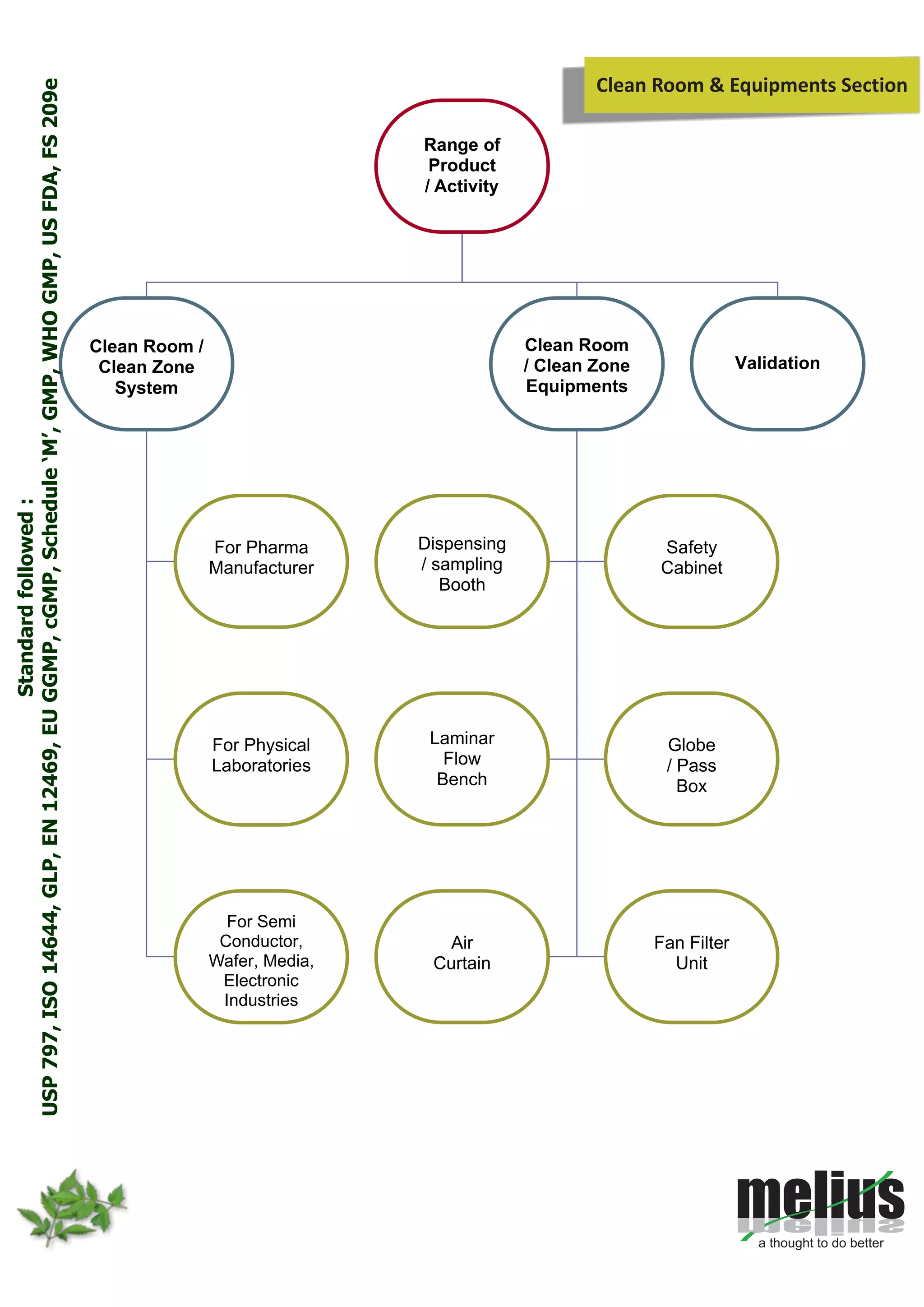
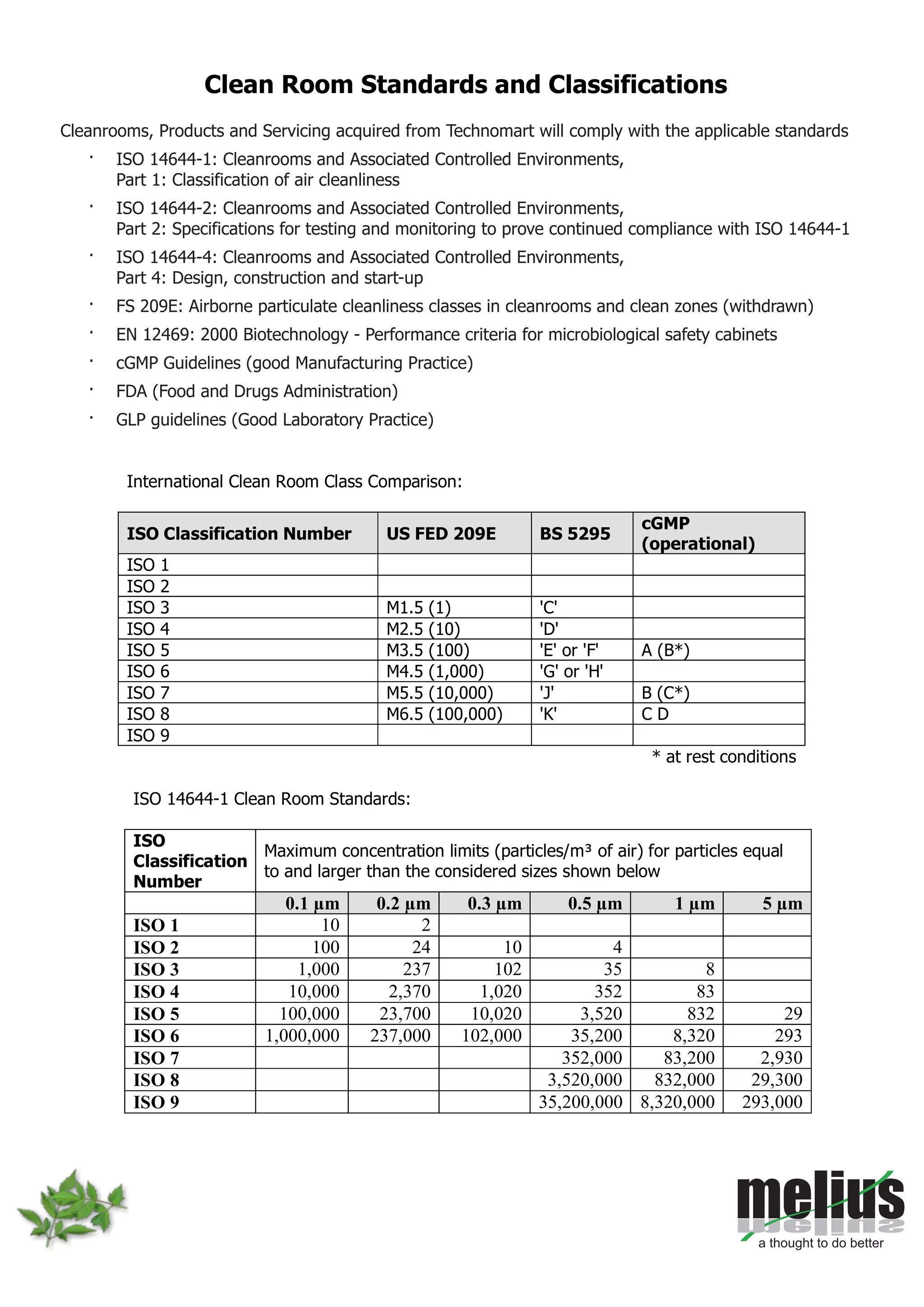
This document discusses Melius's expertise in cleanroom design, construction, and validation for applications in pharmaceuticals, laboratories, and other industries. Melius offers turnkey cleanroom projects from conceptual design to certification, covering a wide range of cleanroom classes from ISO 1 to ISO 9. Services include cleanroom components, separative devices, and testing to standards like ISO 14644 and USP 797. Particle counting is performed to validate cleanroom classifications.