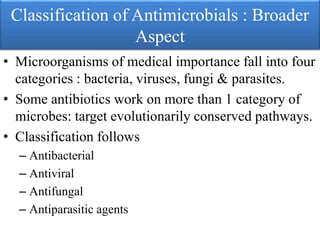
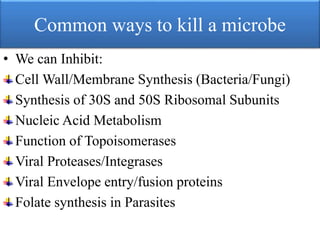
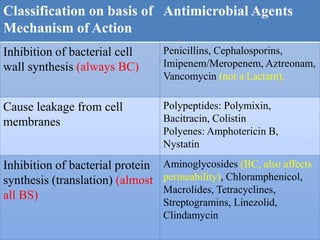
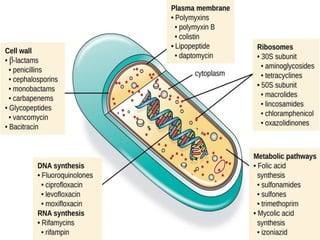
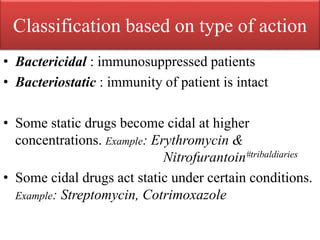
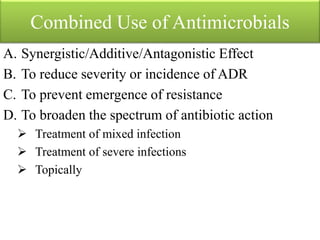
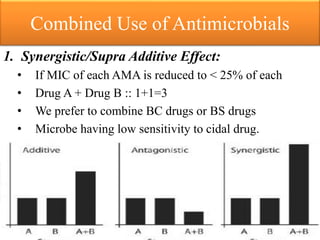
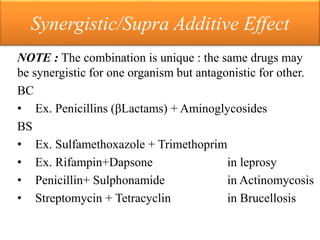
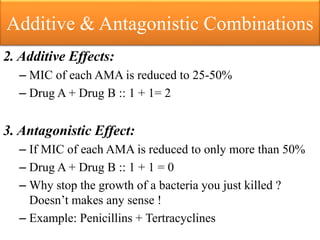
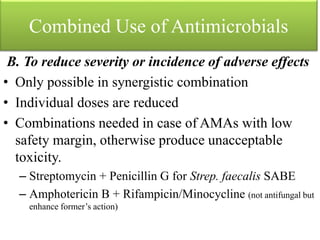
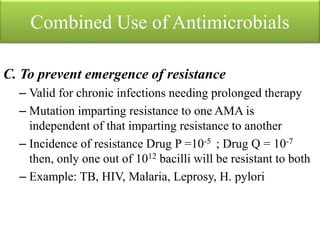
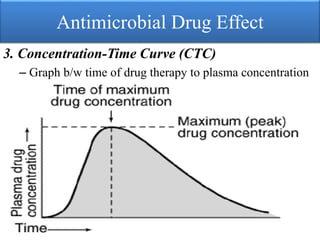
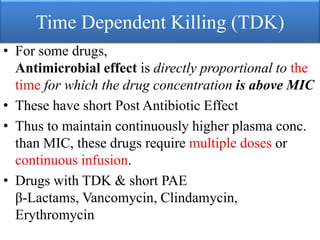
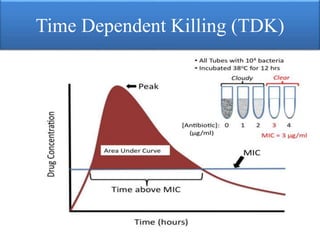
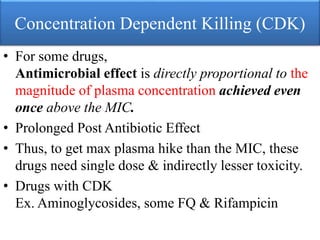
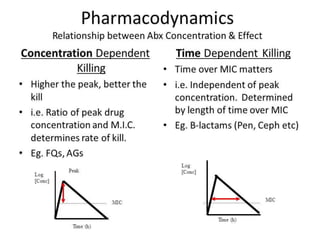
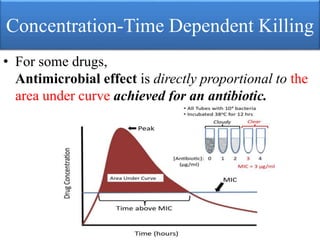
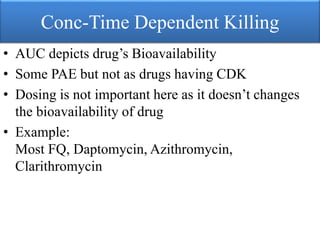
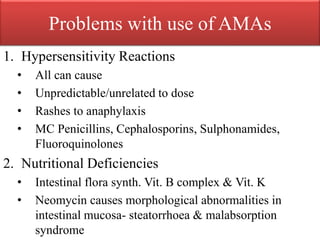
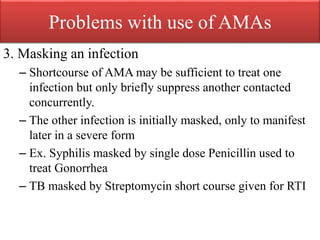
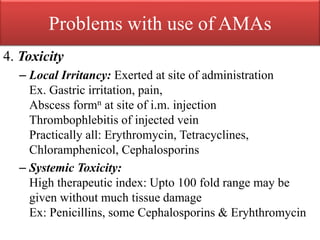
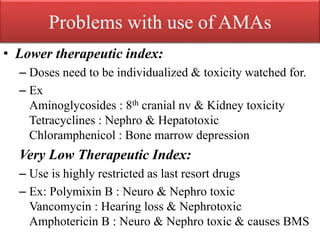
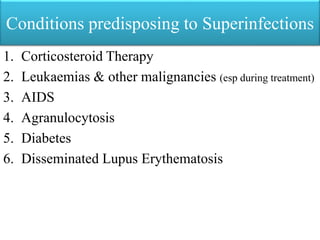
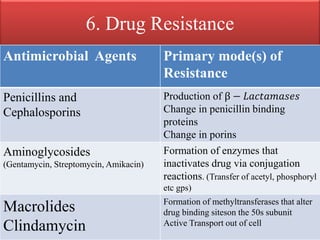
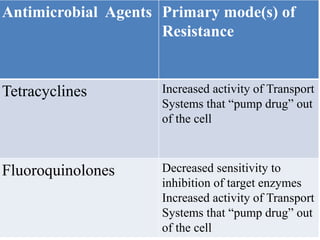
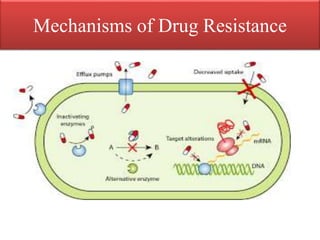
This document provides an introduction to antimicrobial drugs. It discusses the objectives of the lecture which include providing a historical review, definitions, and classifications of antimicrobial drugs. It covers ways that microbes can be killed and how antimicrobial drugs are classified based on their chemical structure, mechanism of action, spectrum of activity, and type of action. Combinations of antimicrobial drugs and their effects, as well as factors influencing drug effects like minimal inhibitory concentration and post-antibiotic effect, are summarized.