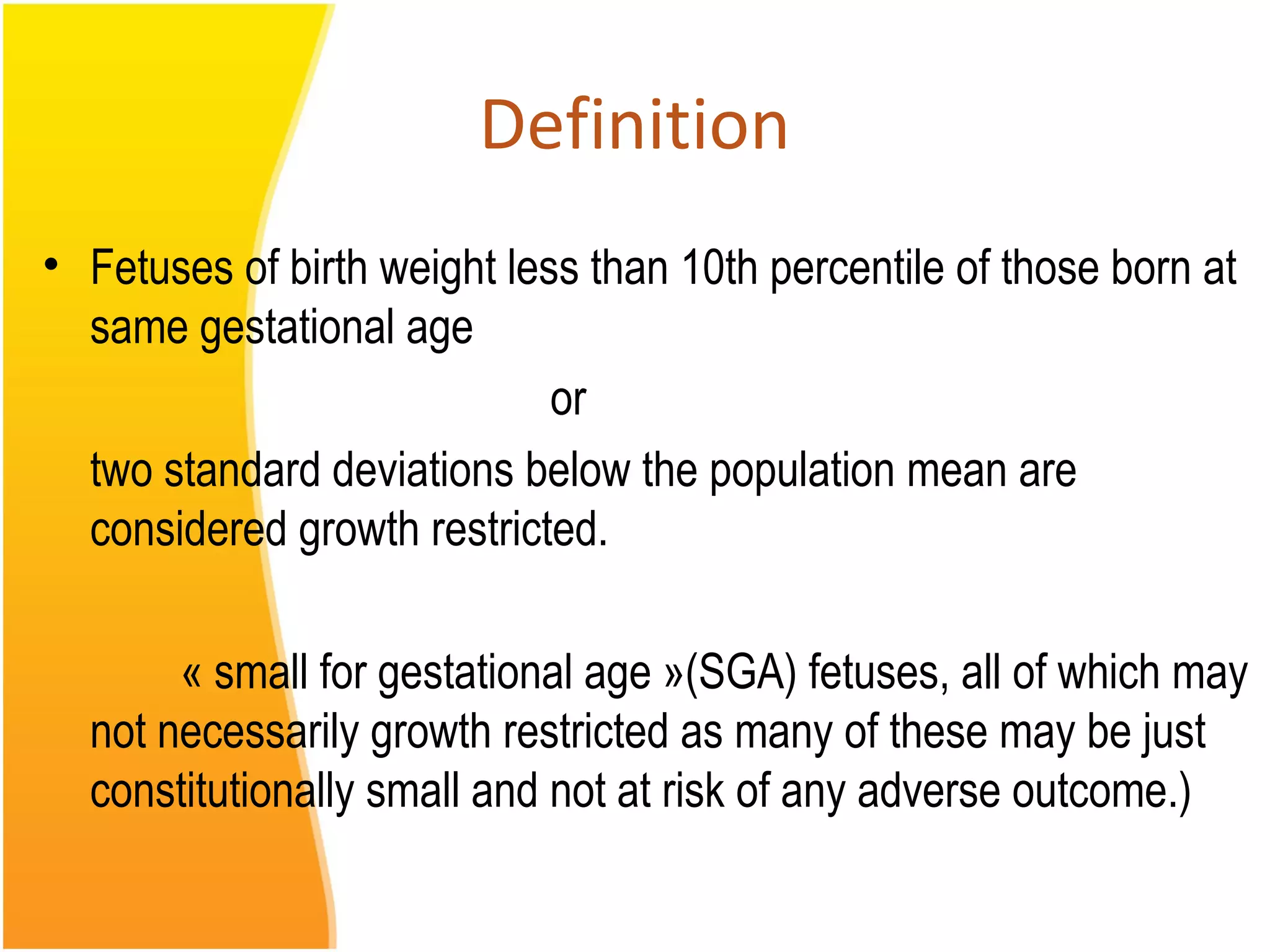
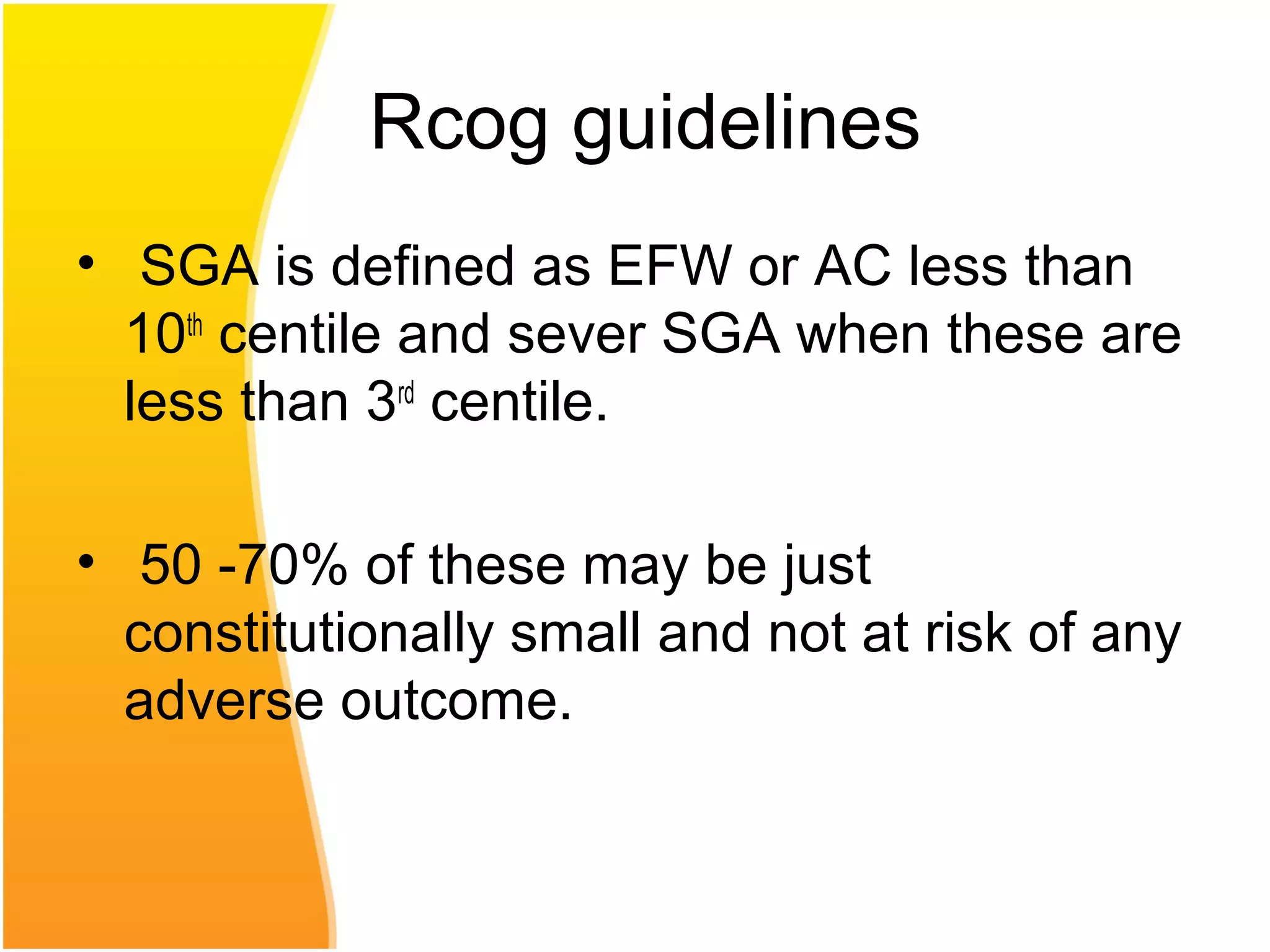
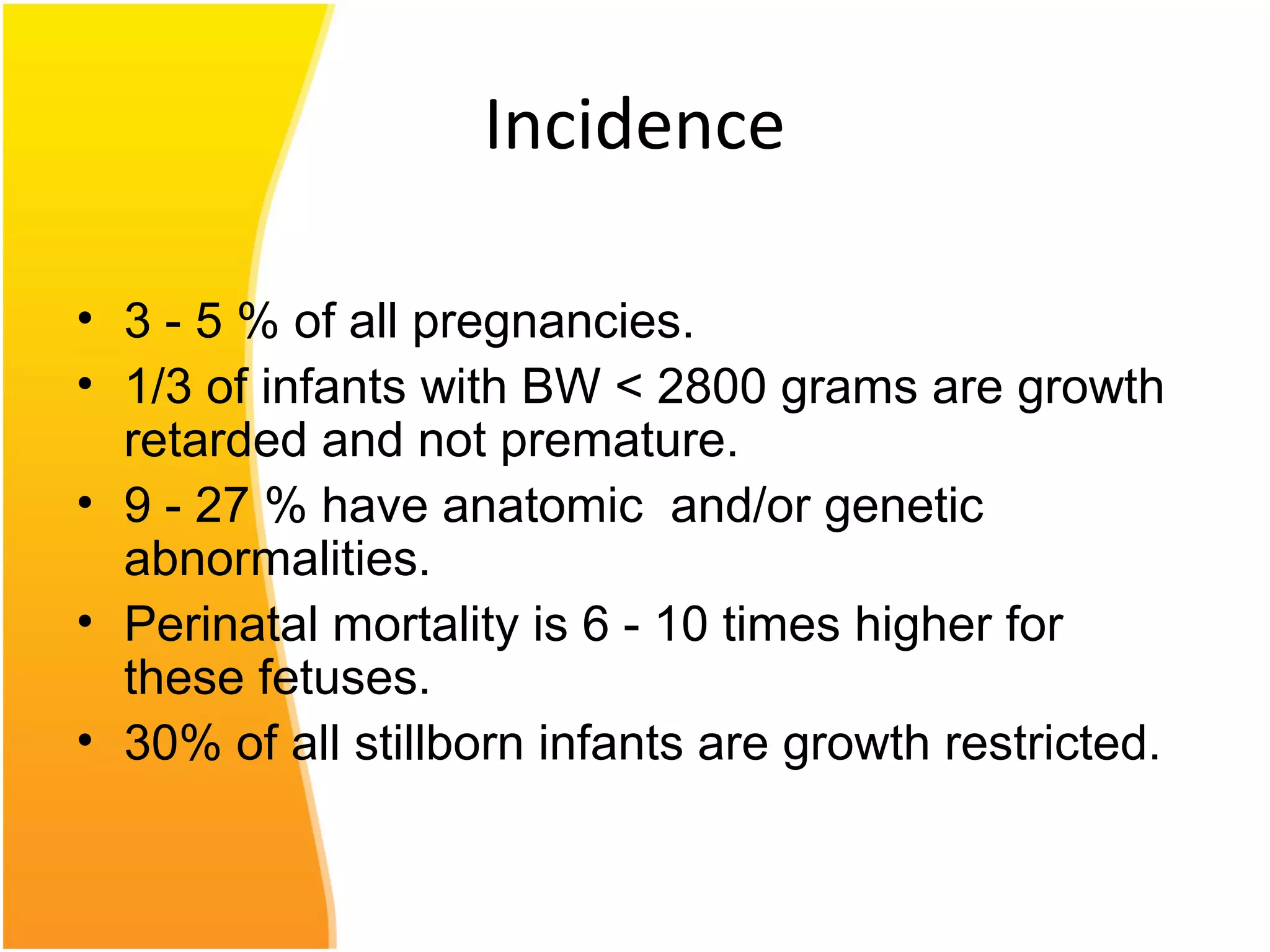
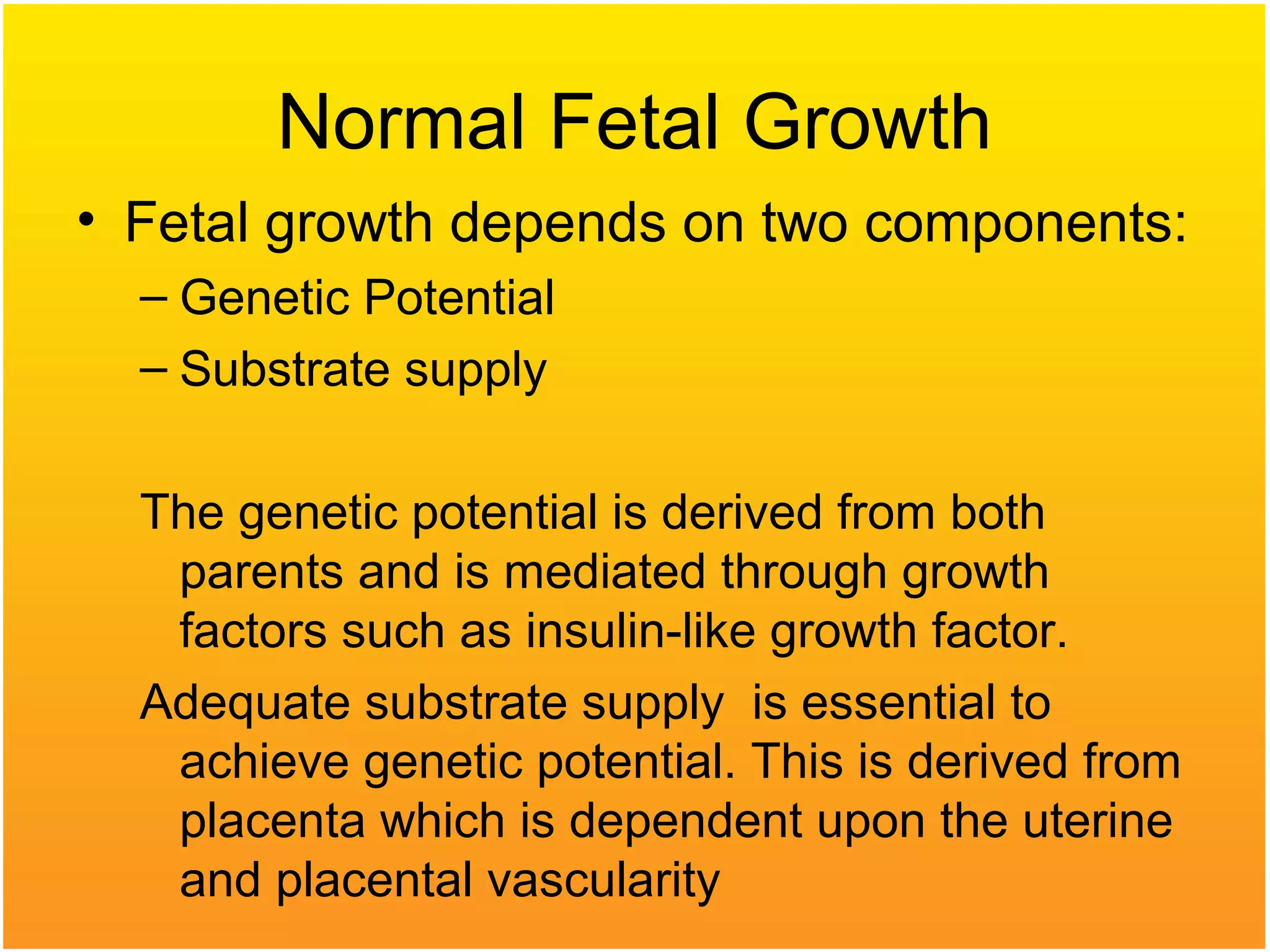
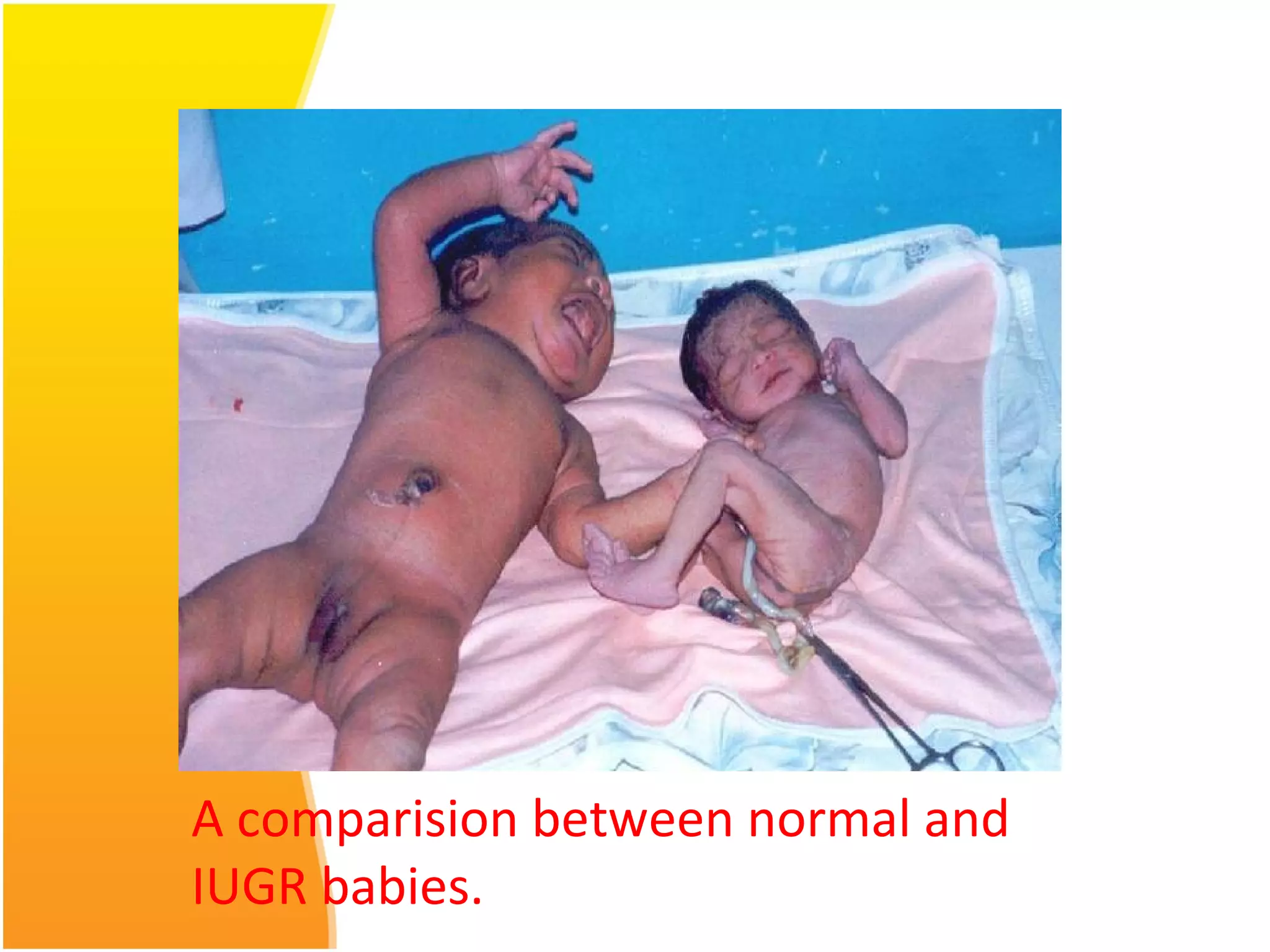
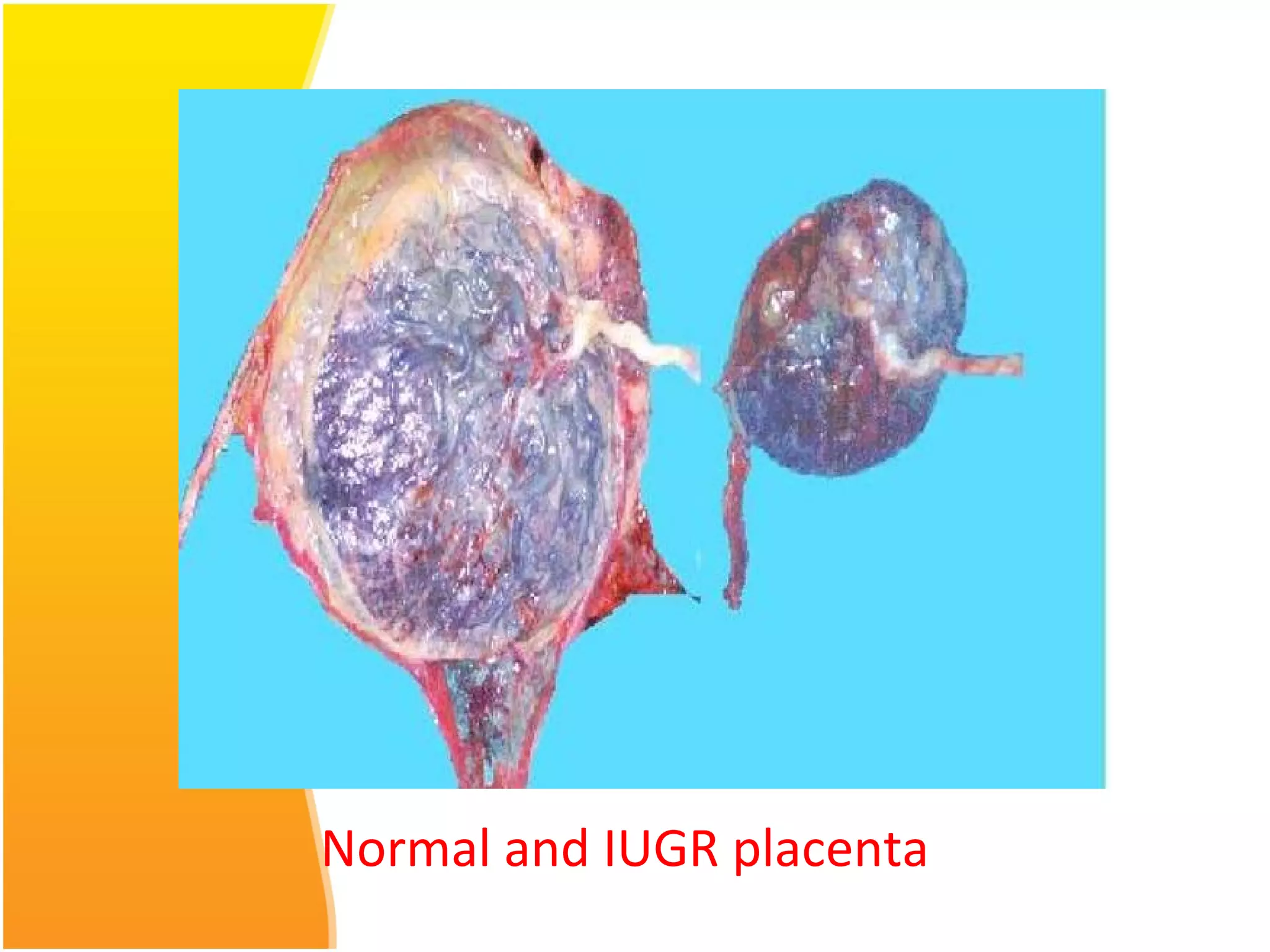
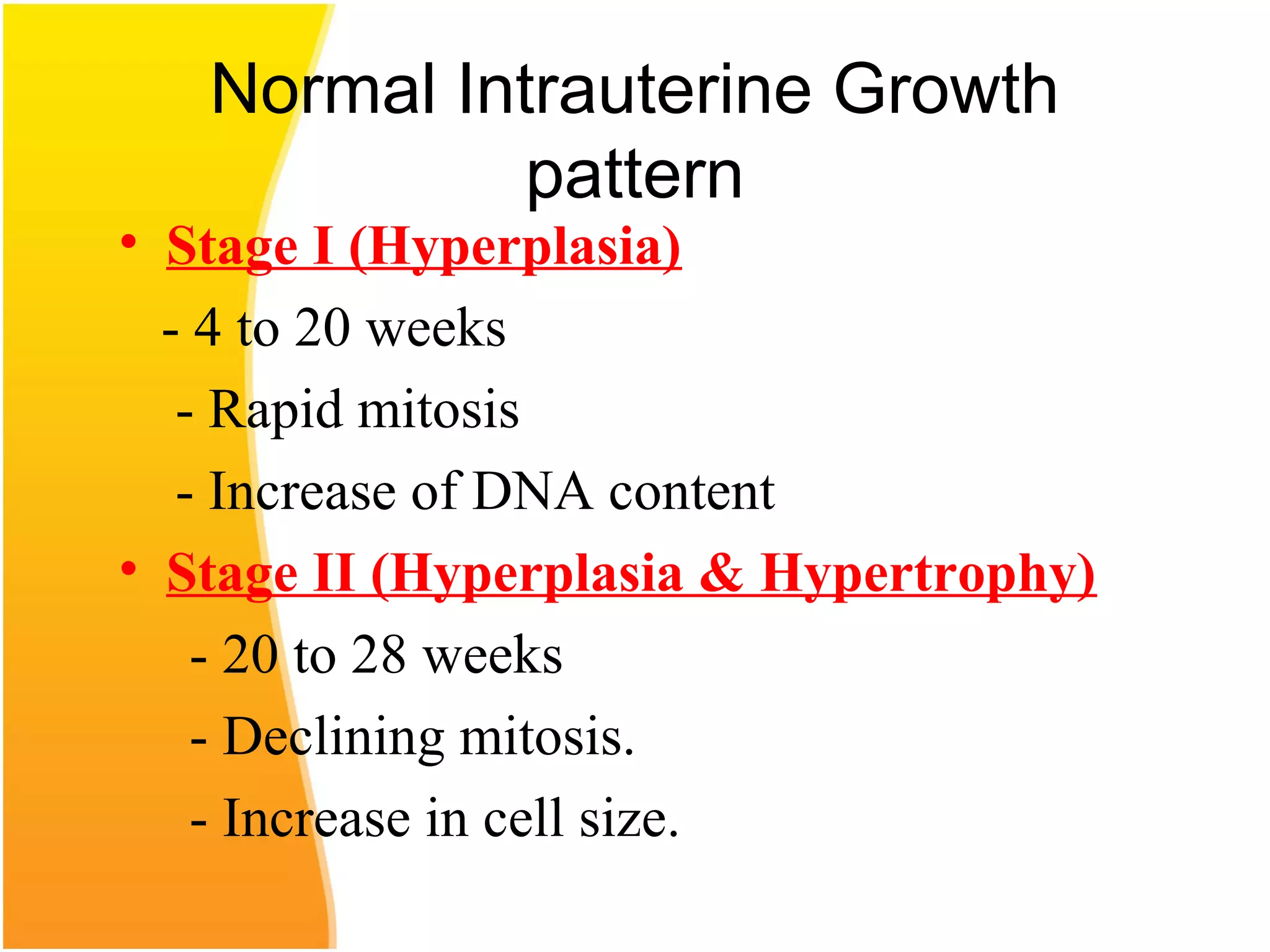
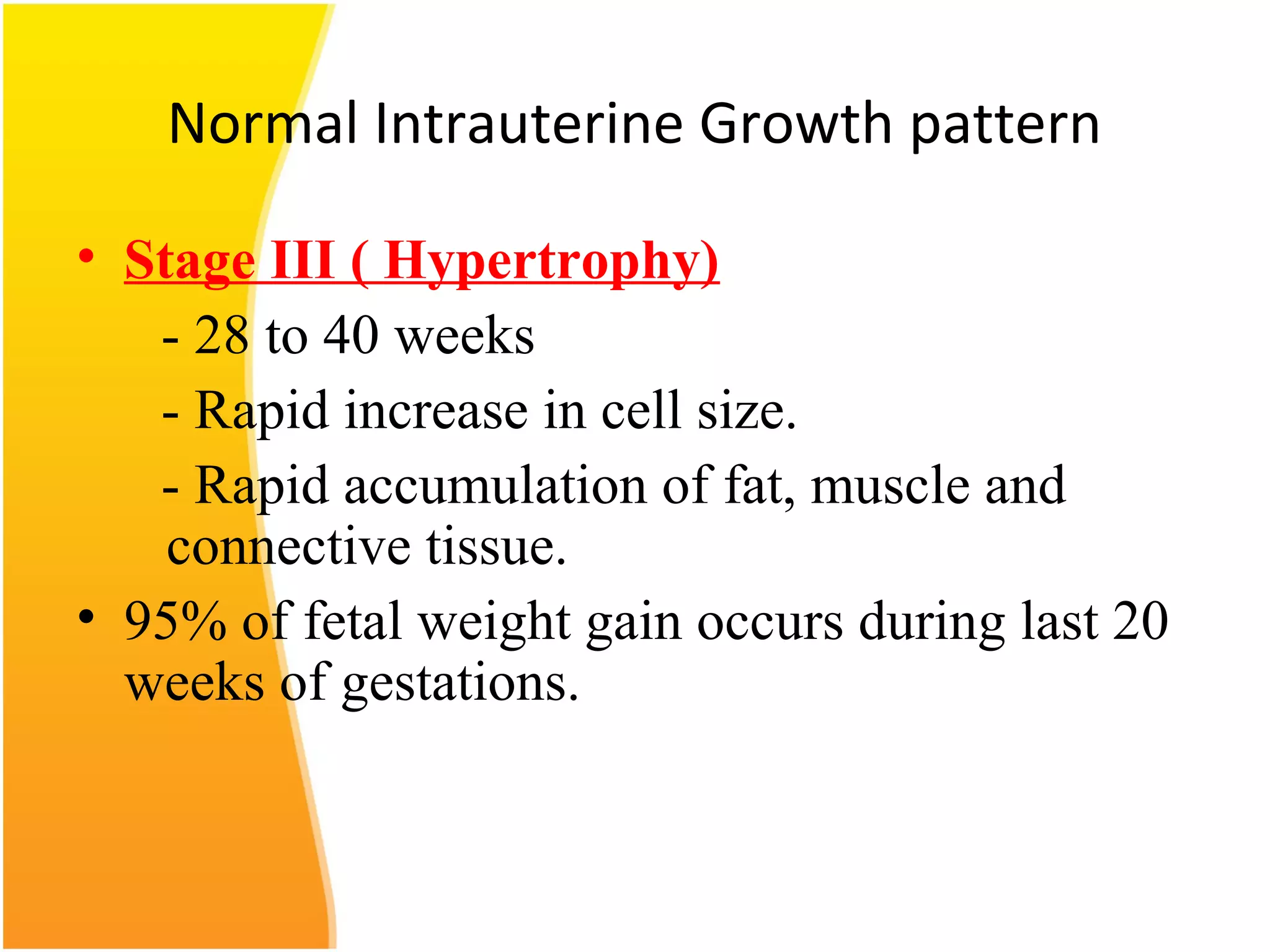
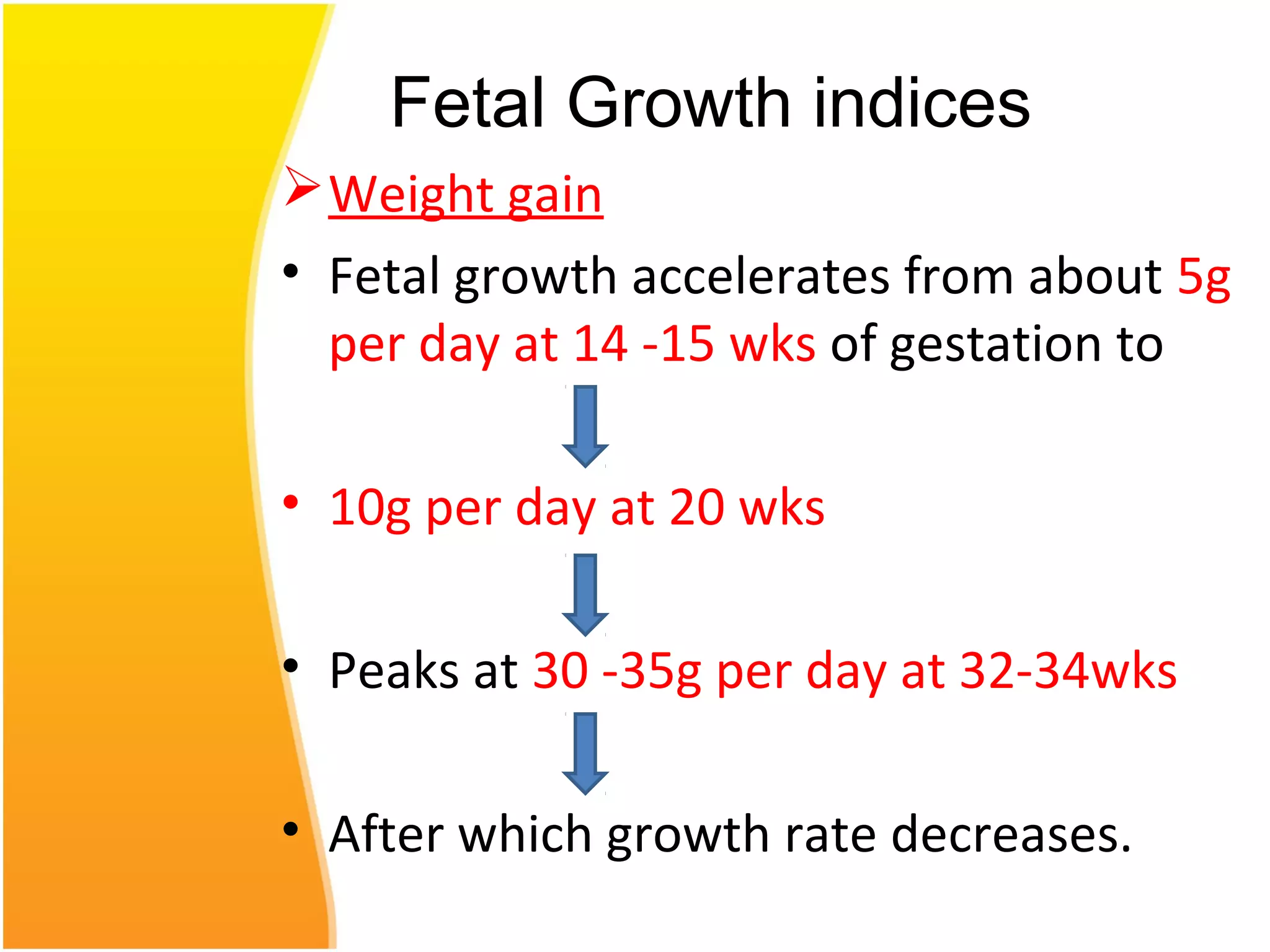
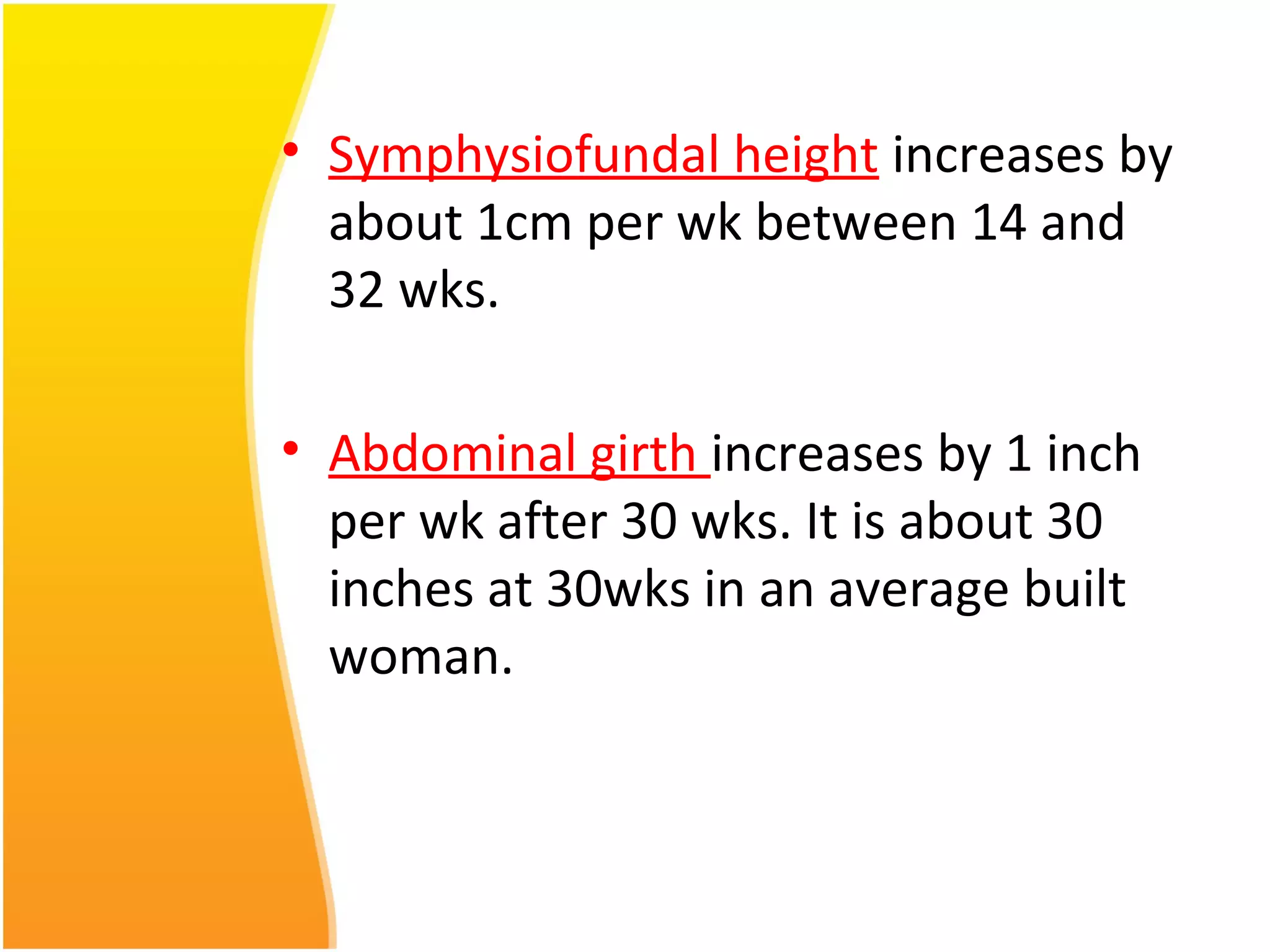
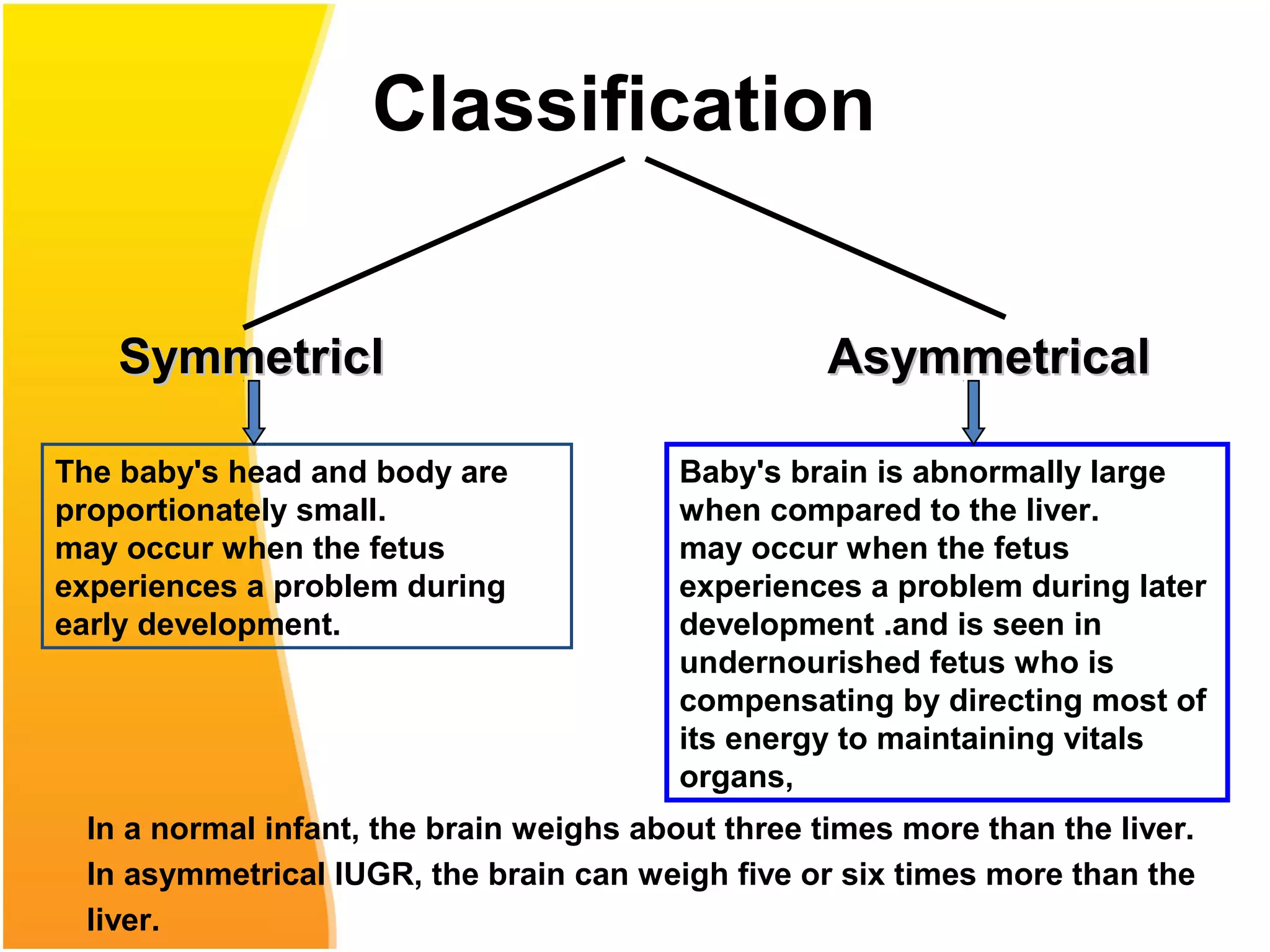
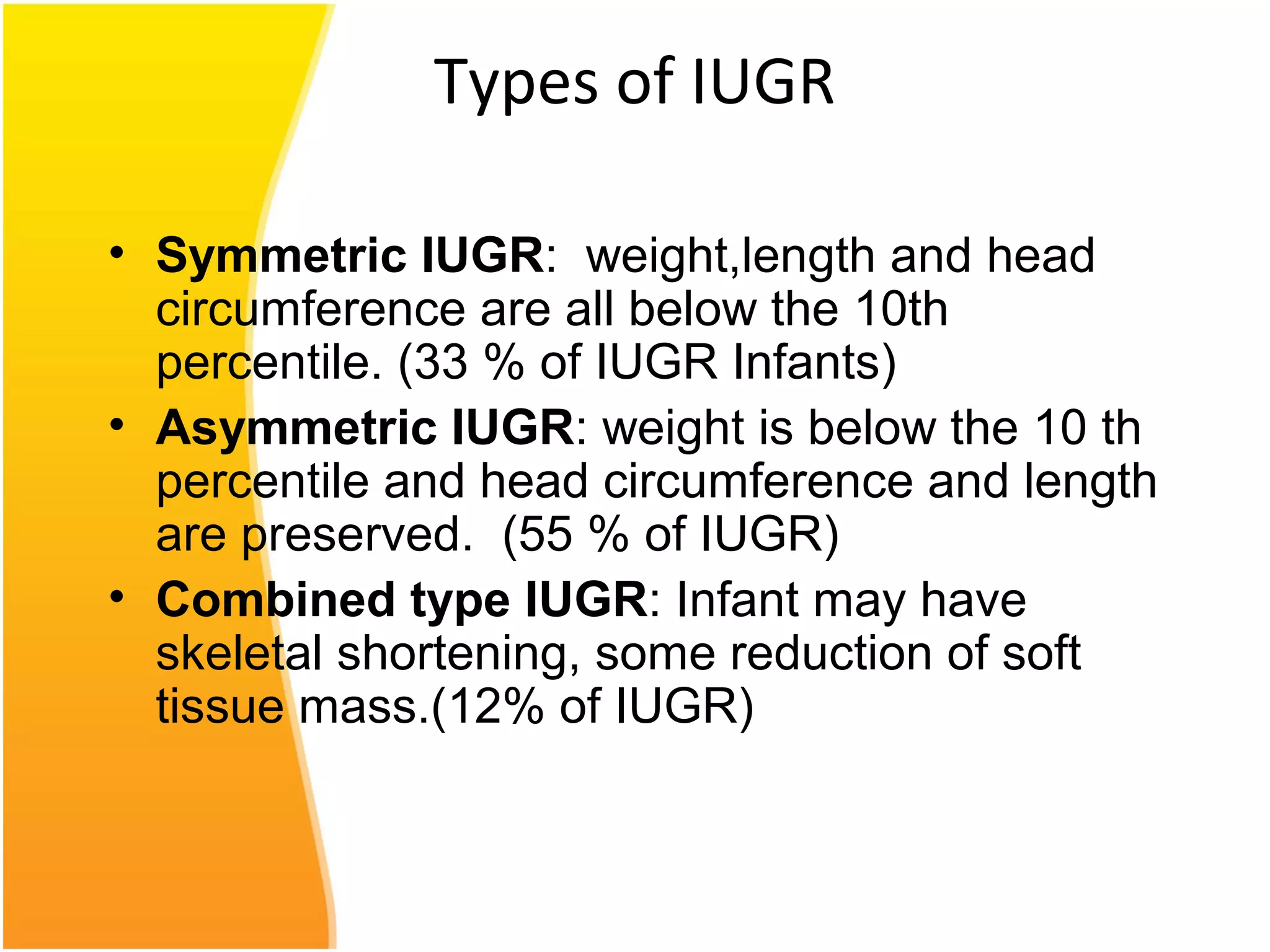
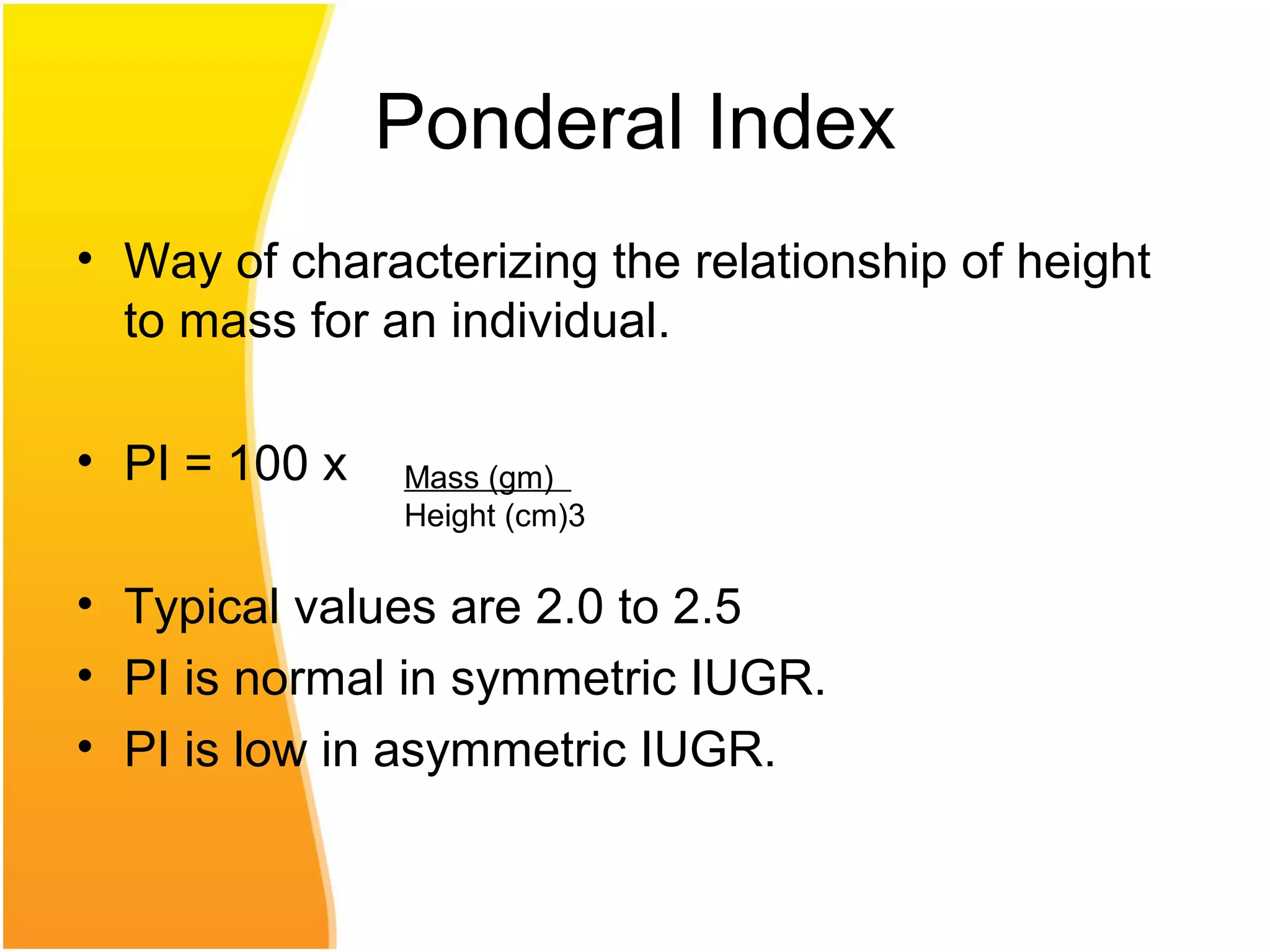
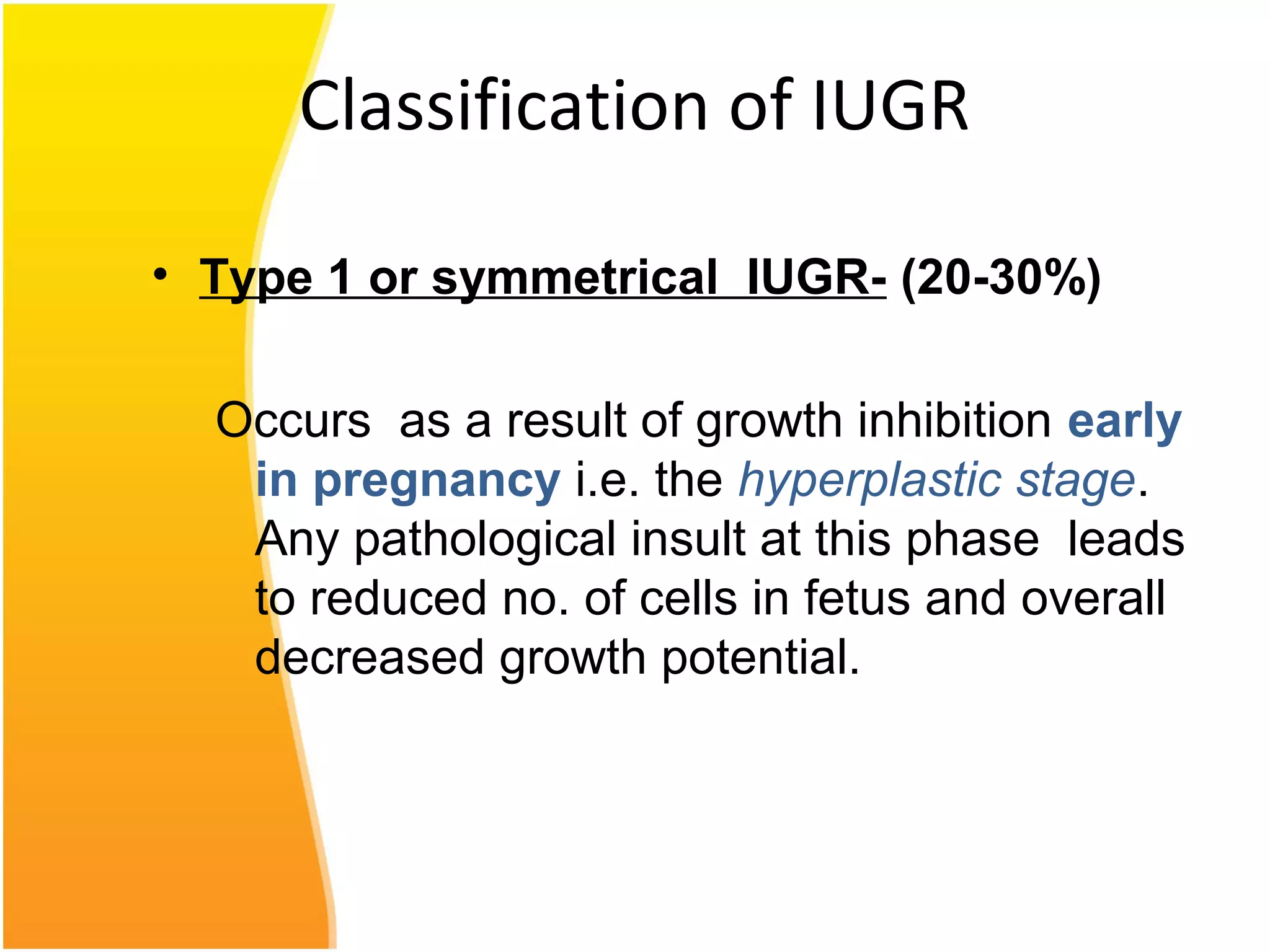
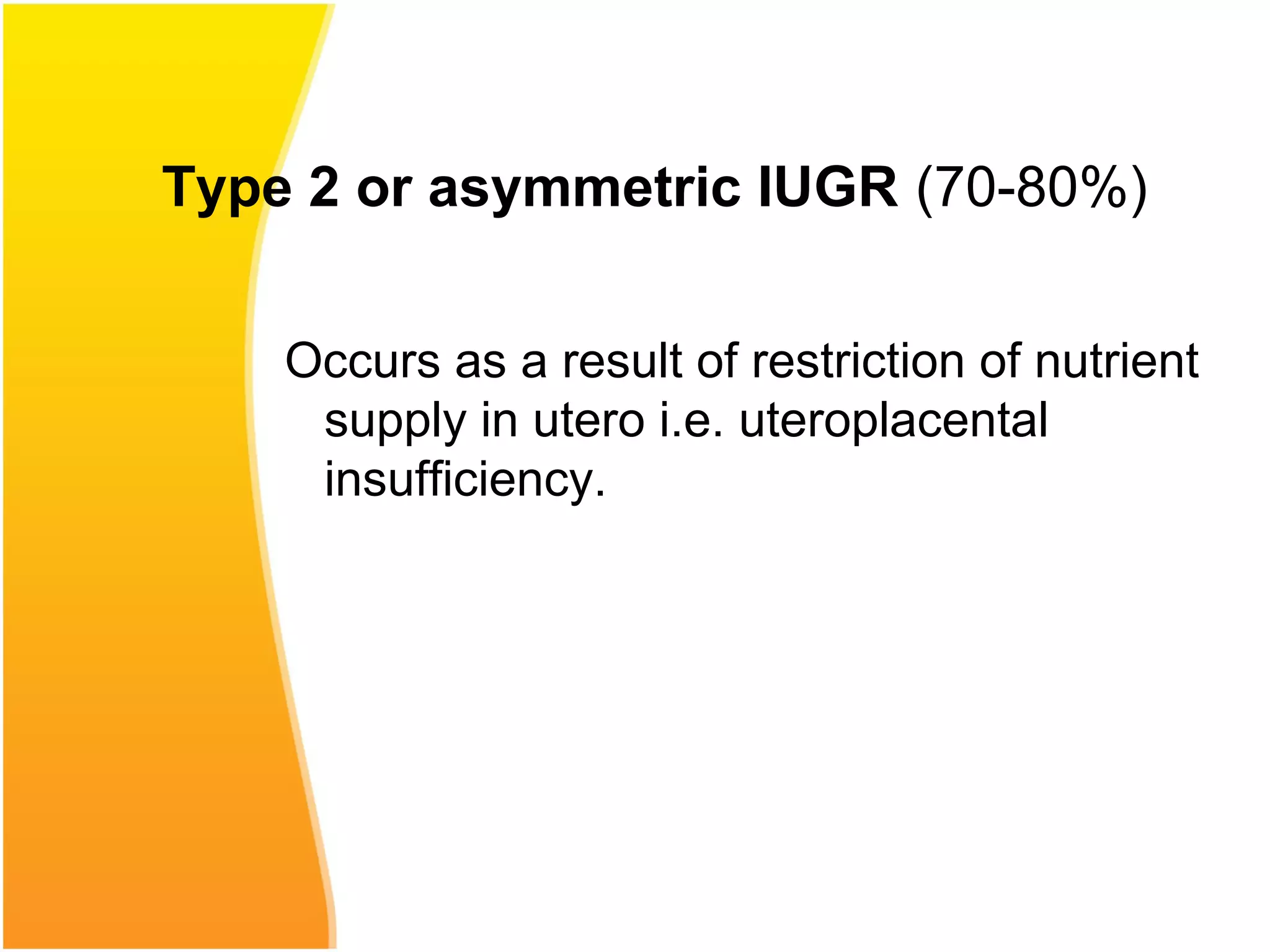
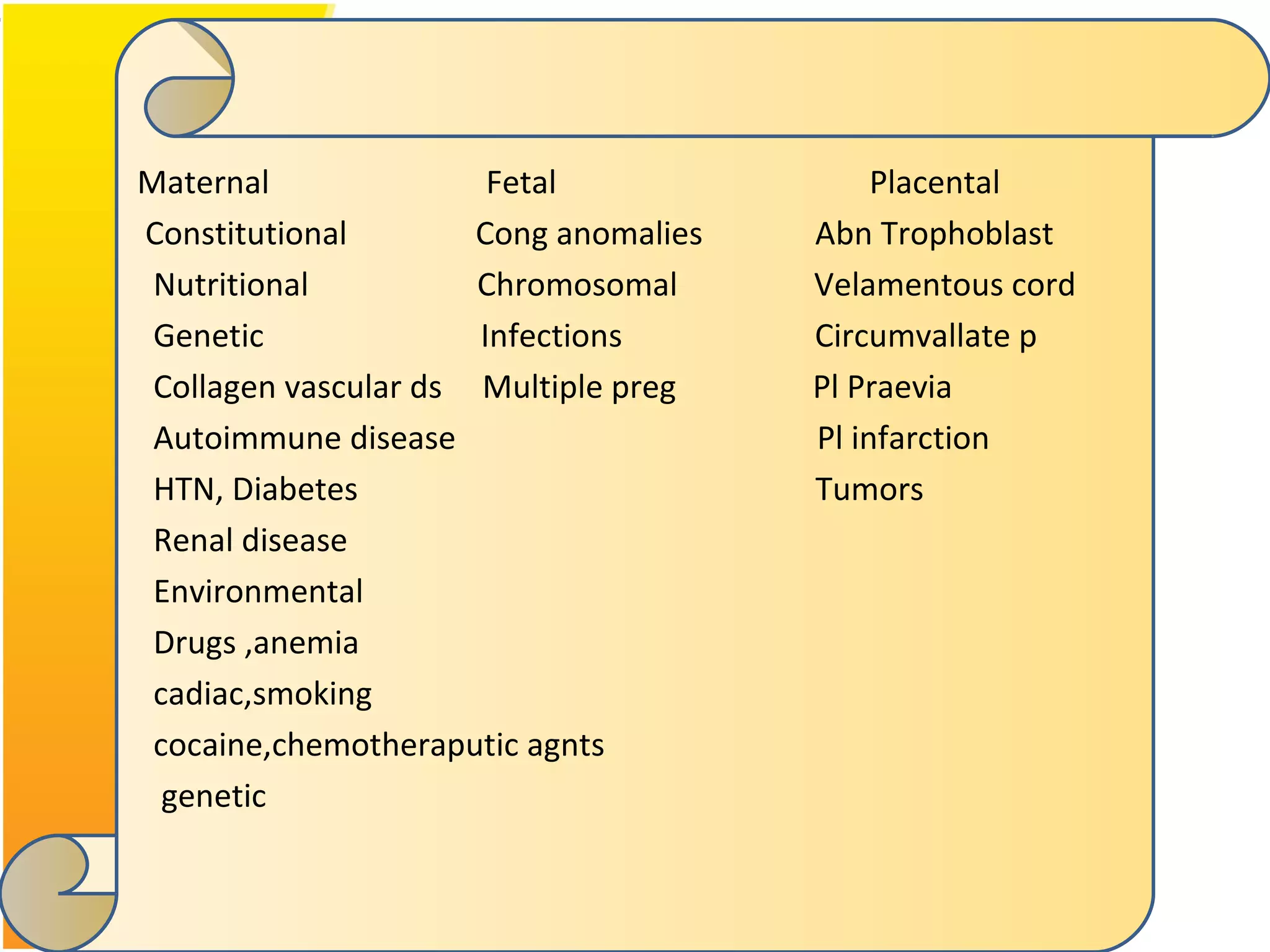
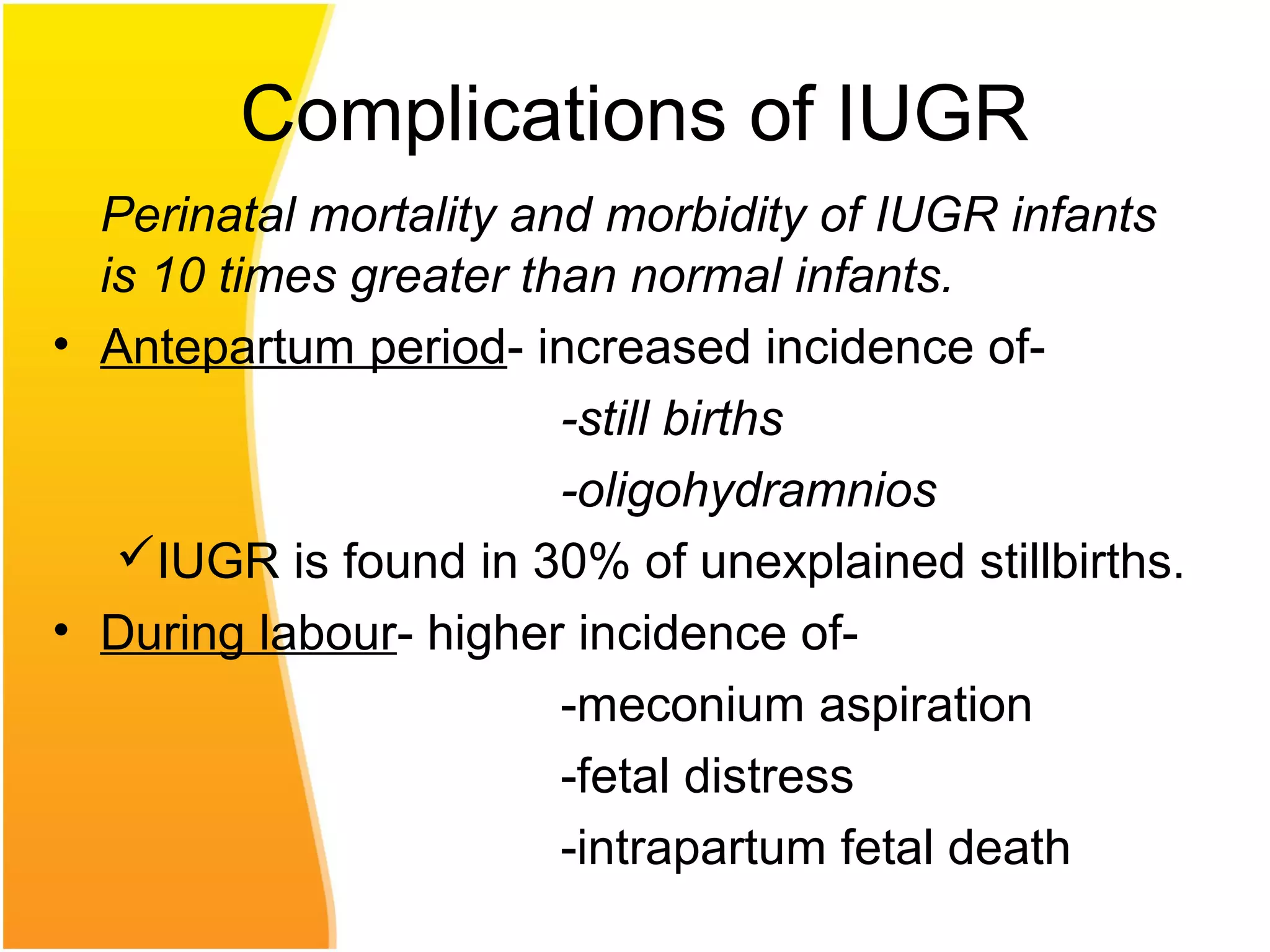
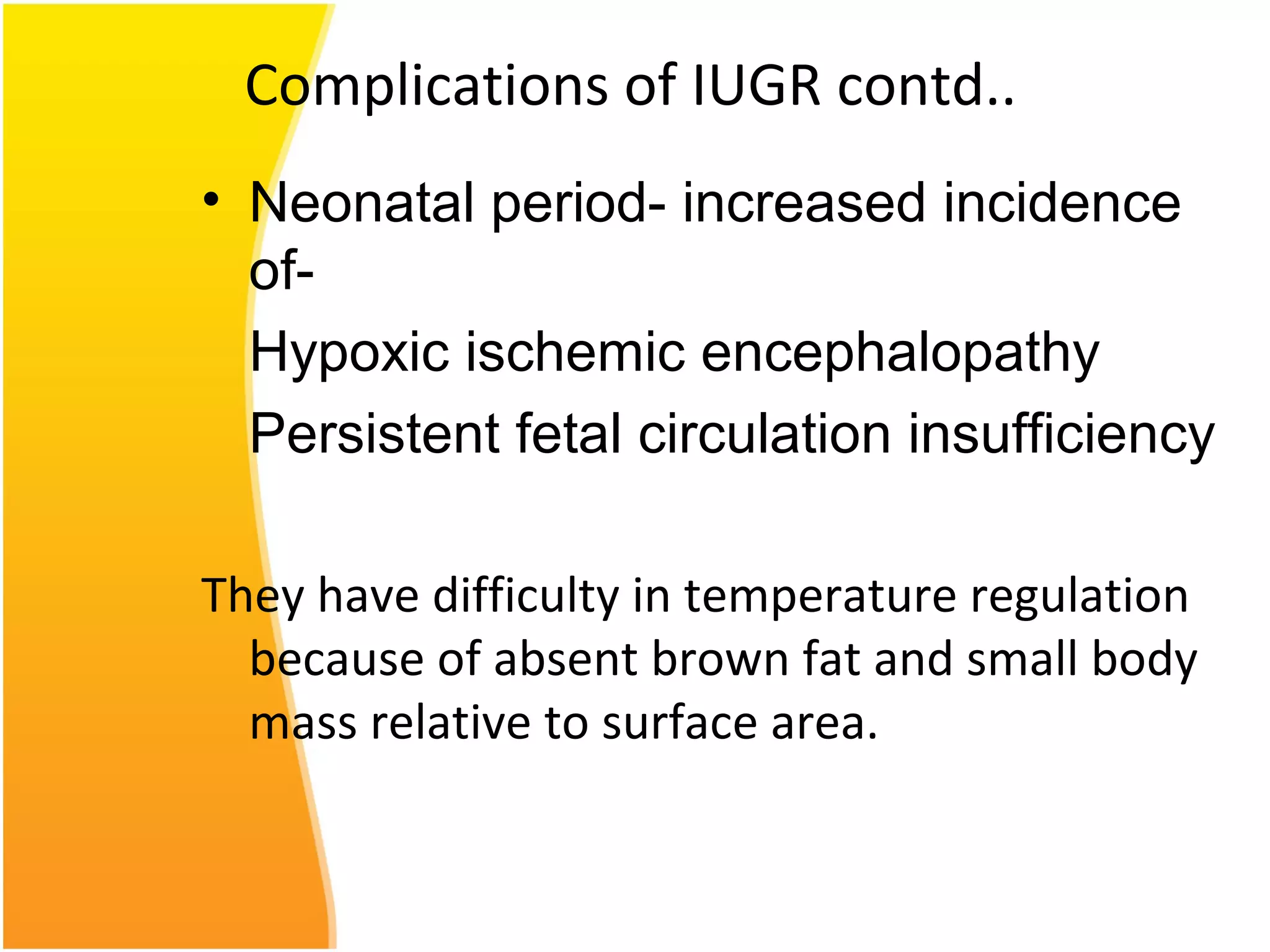
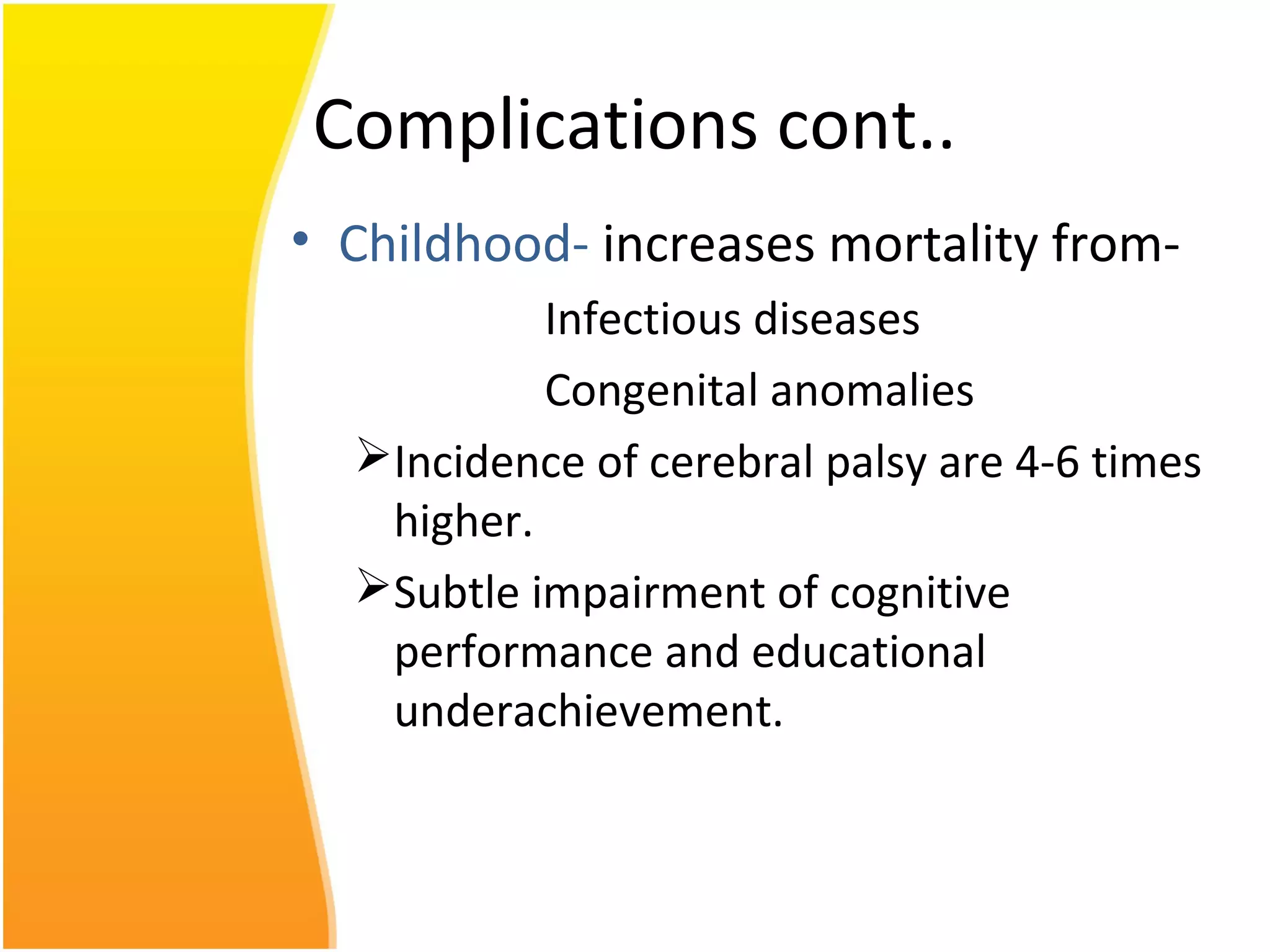
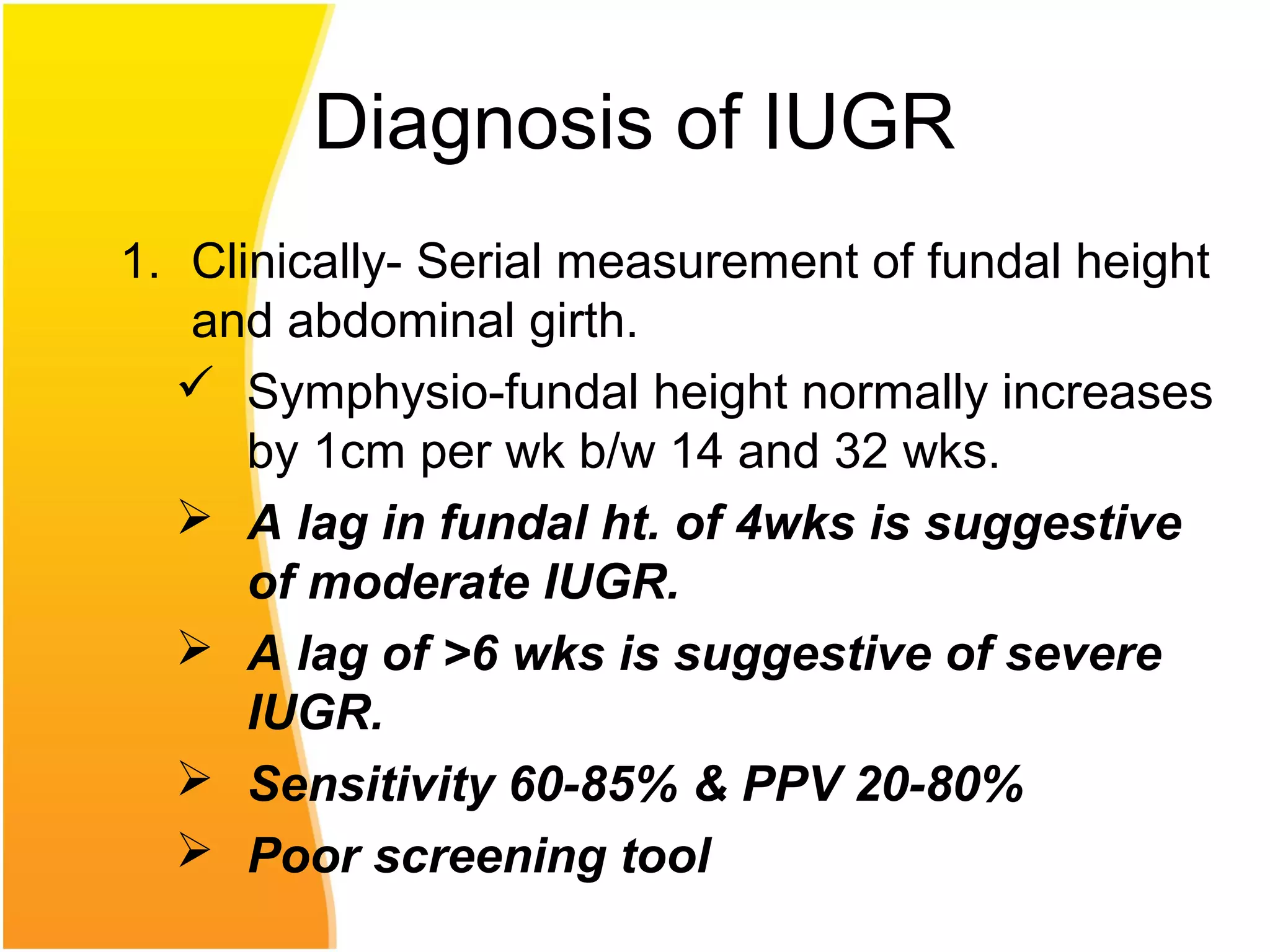
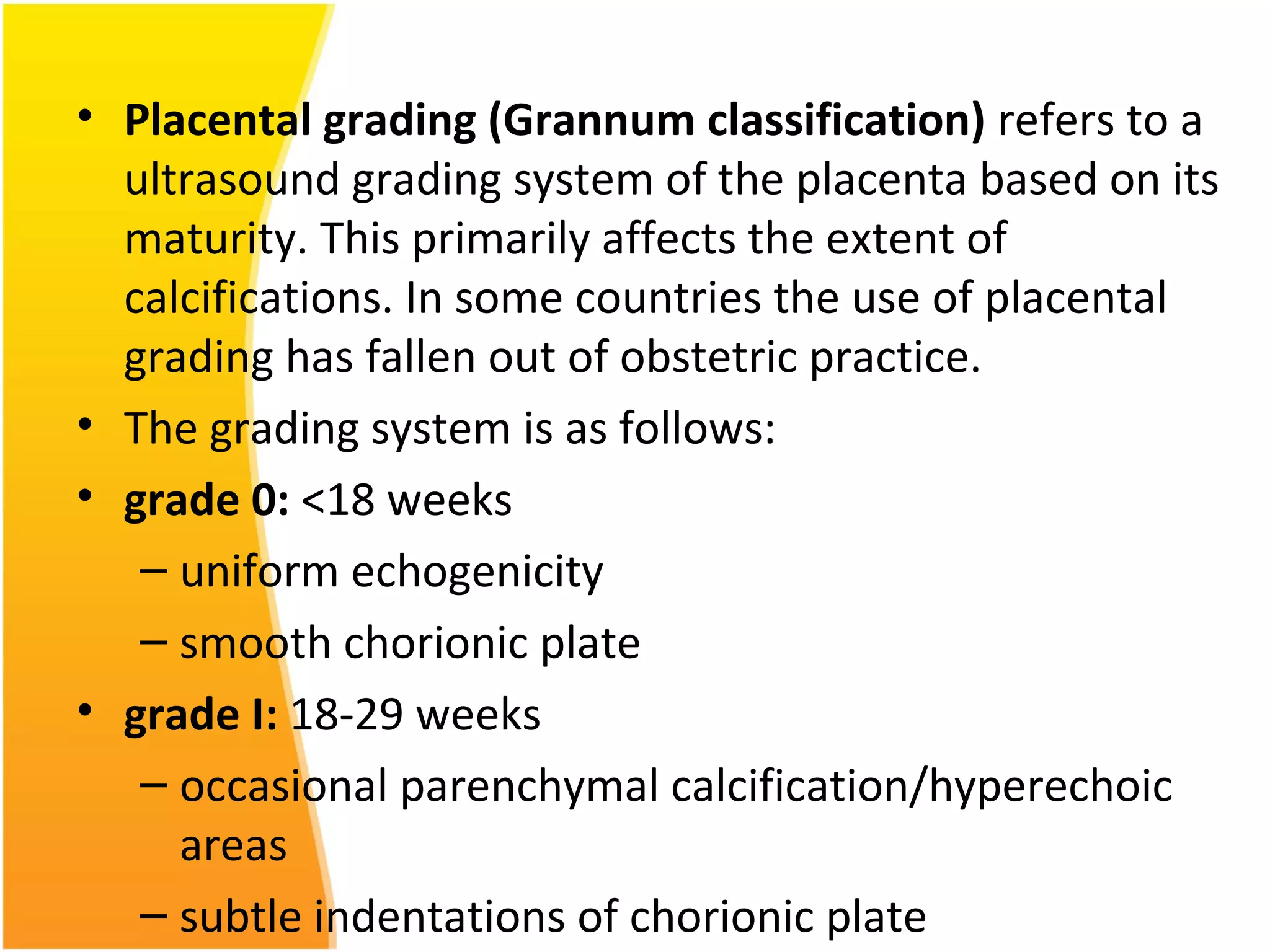
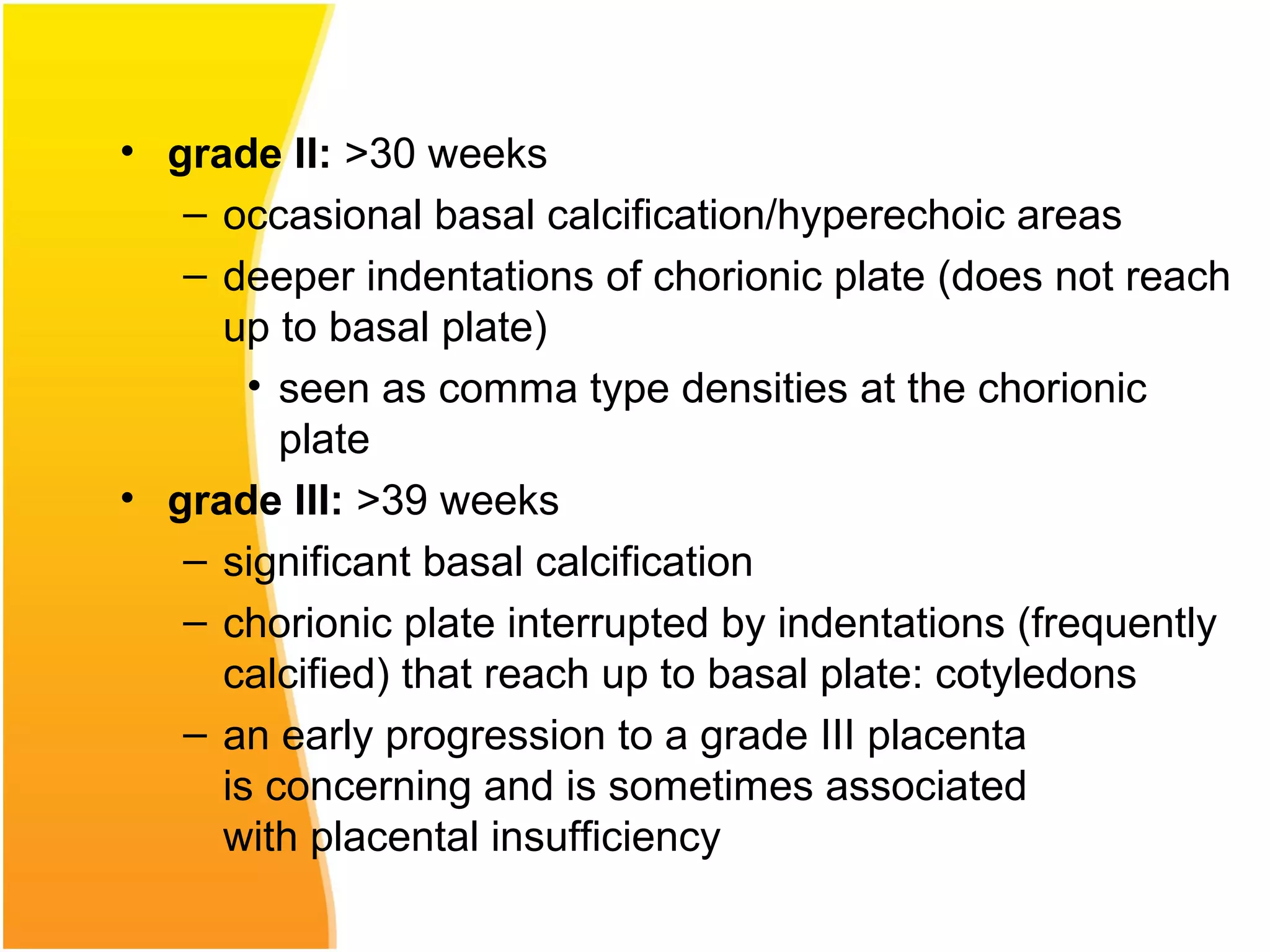
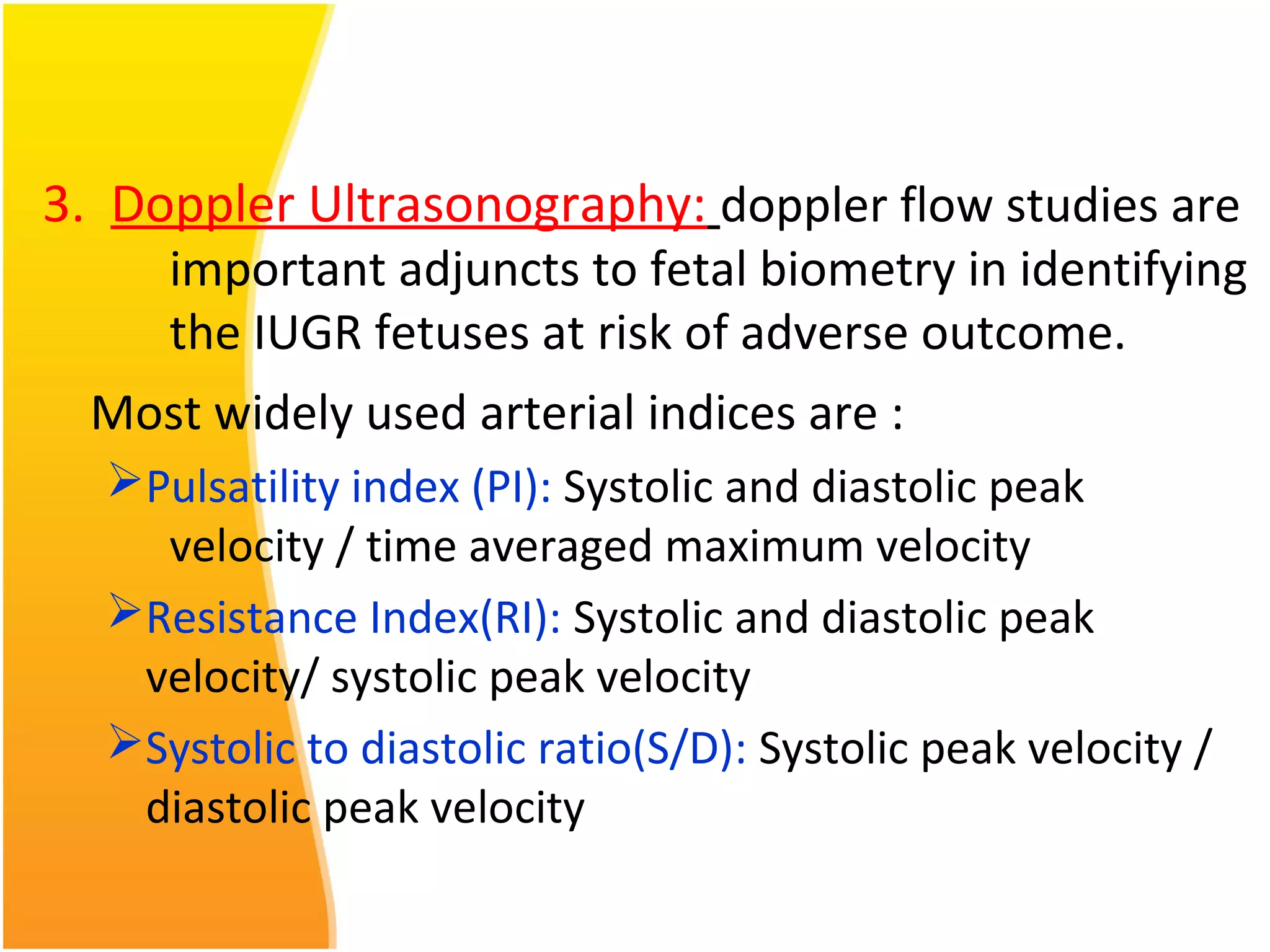
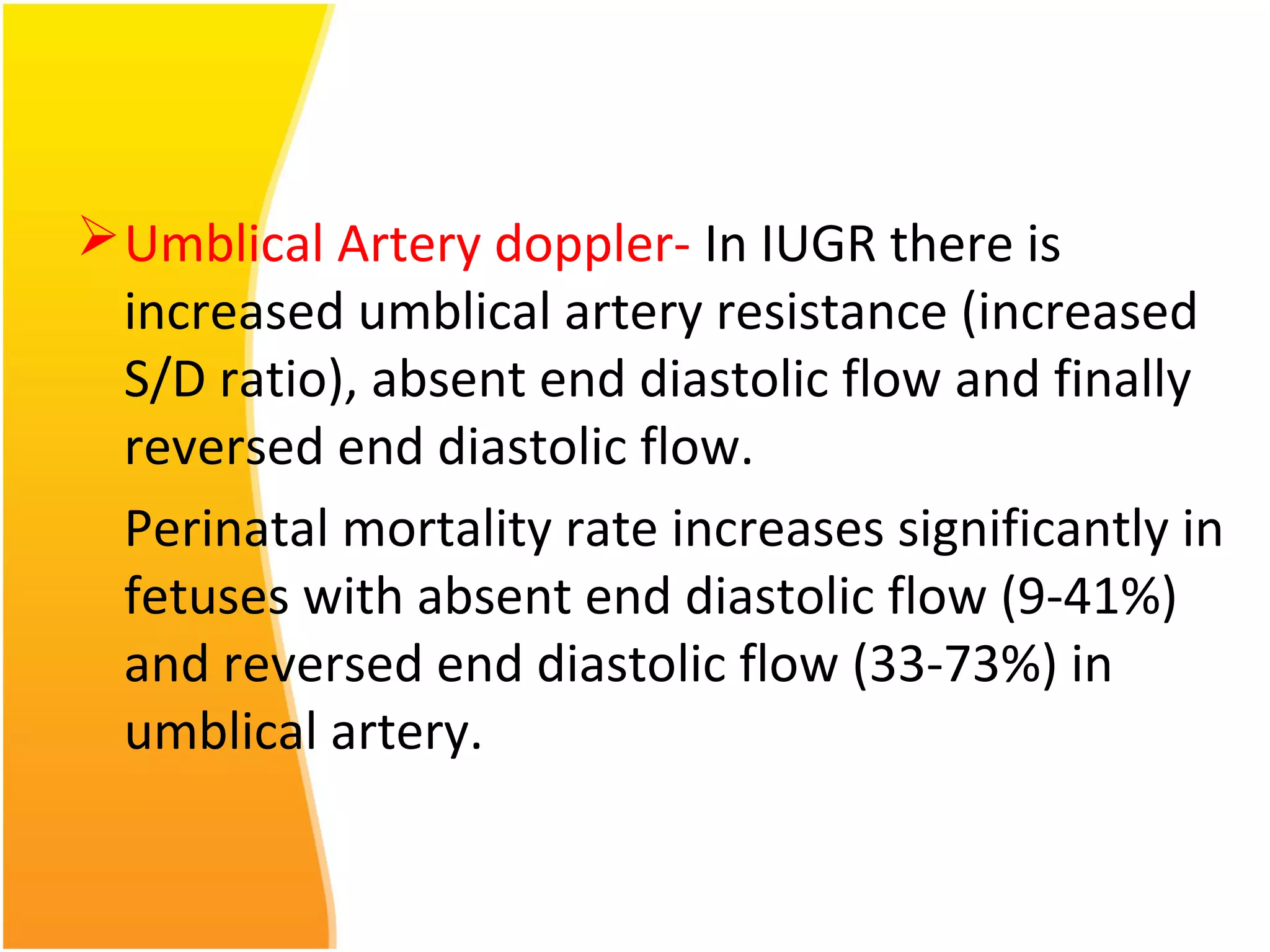
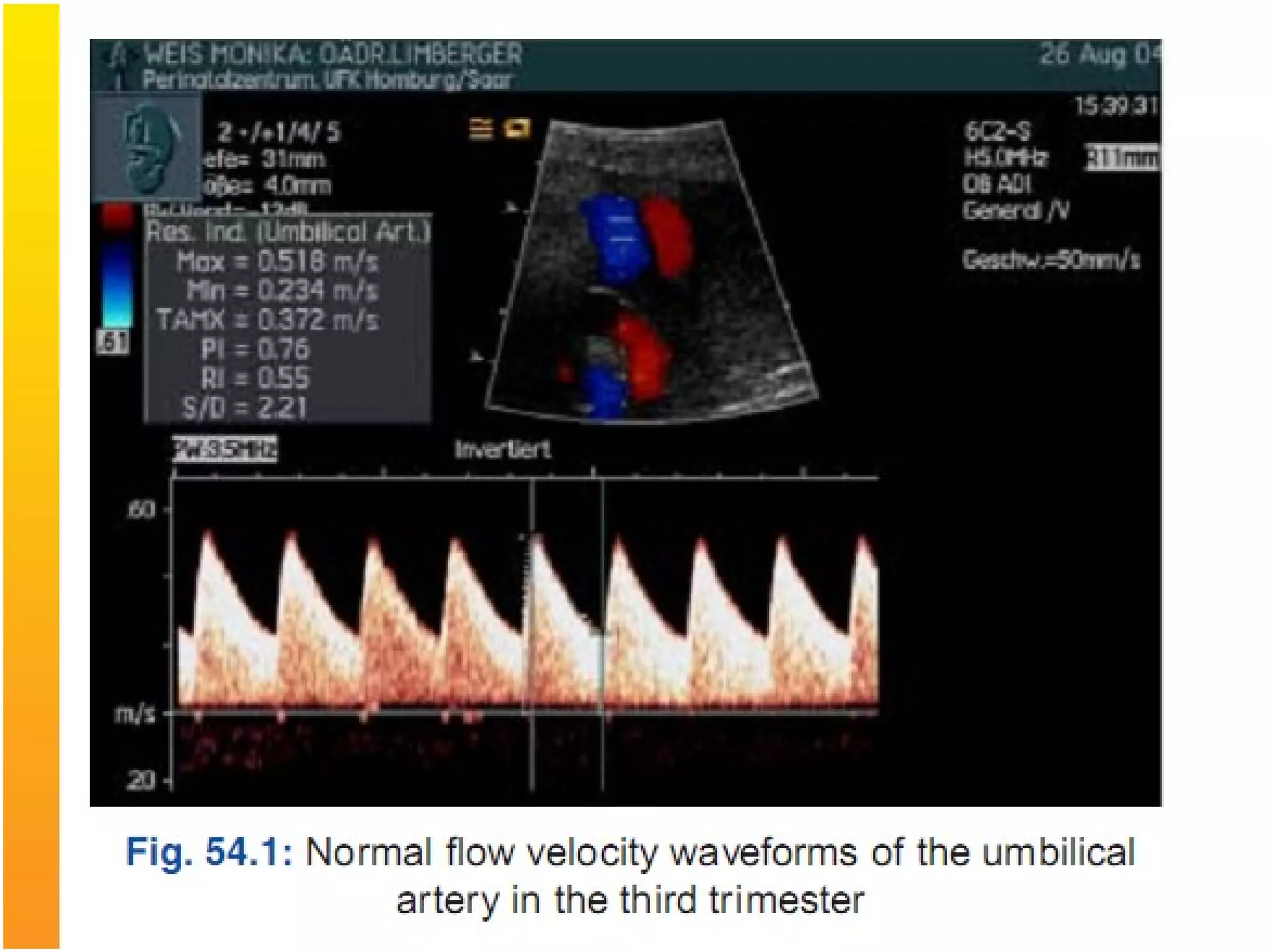
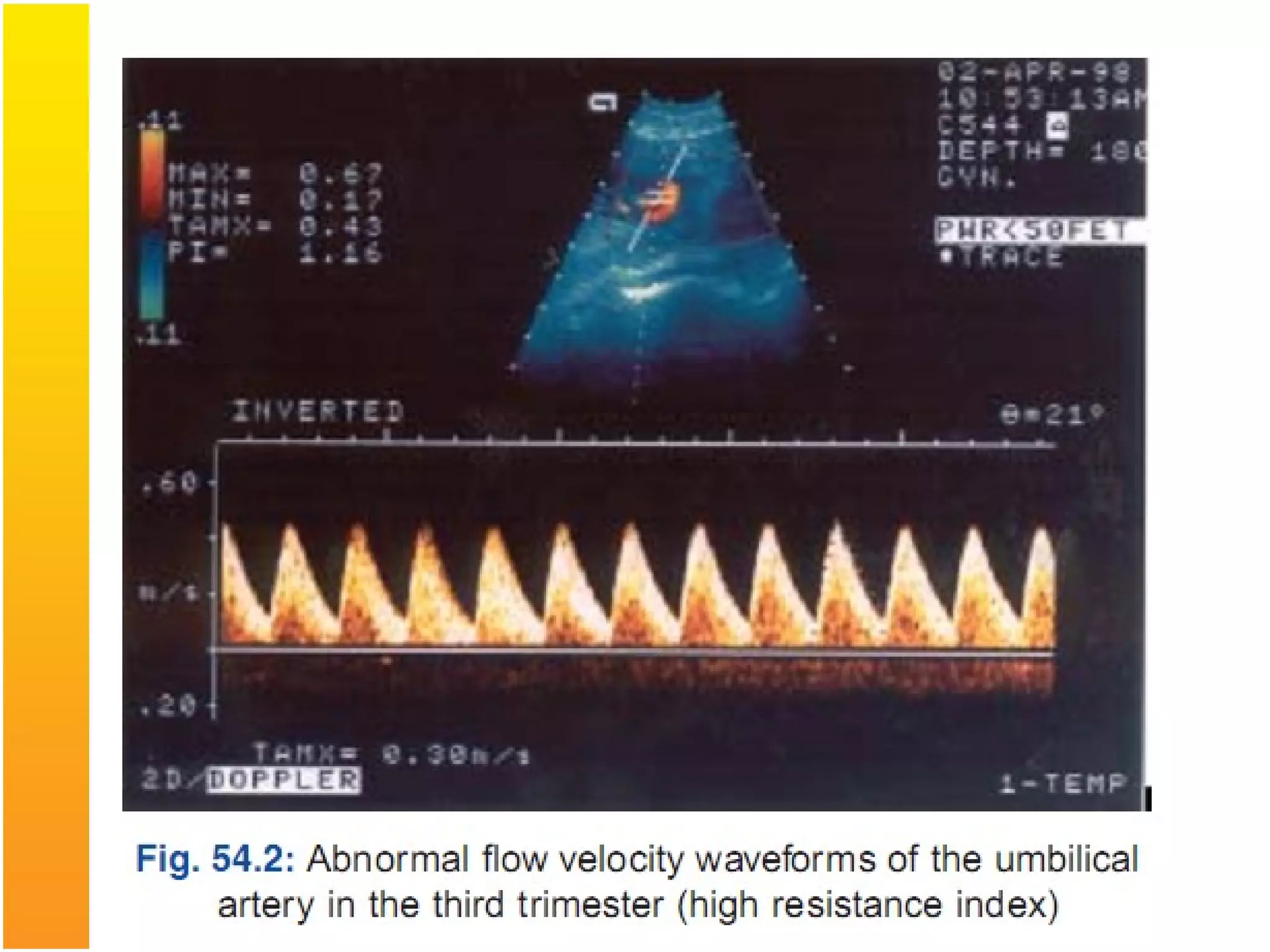
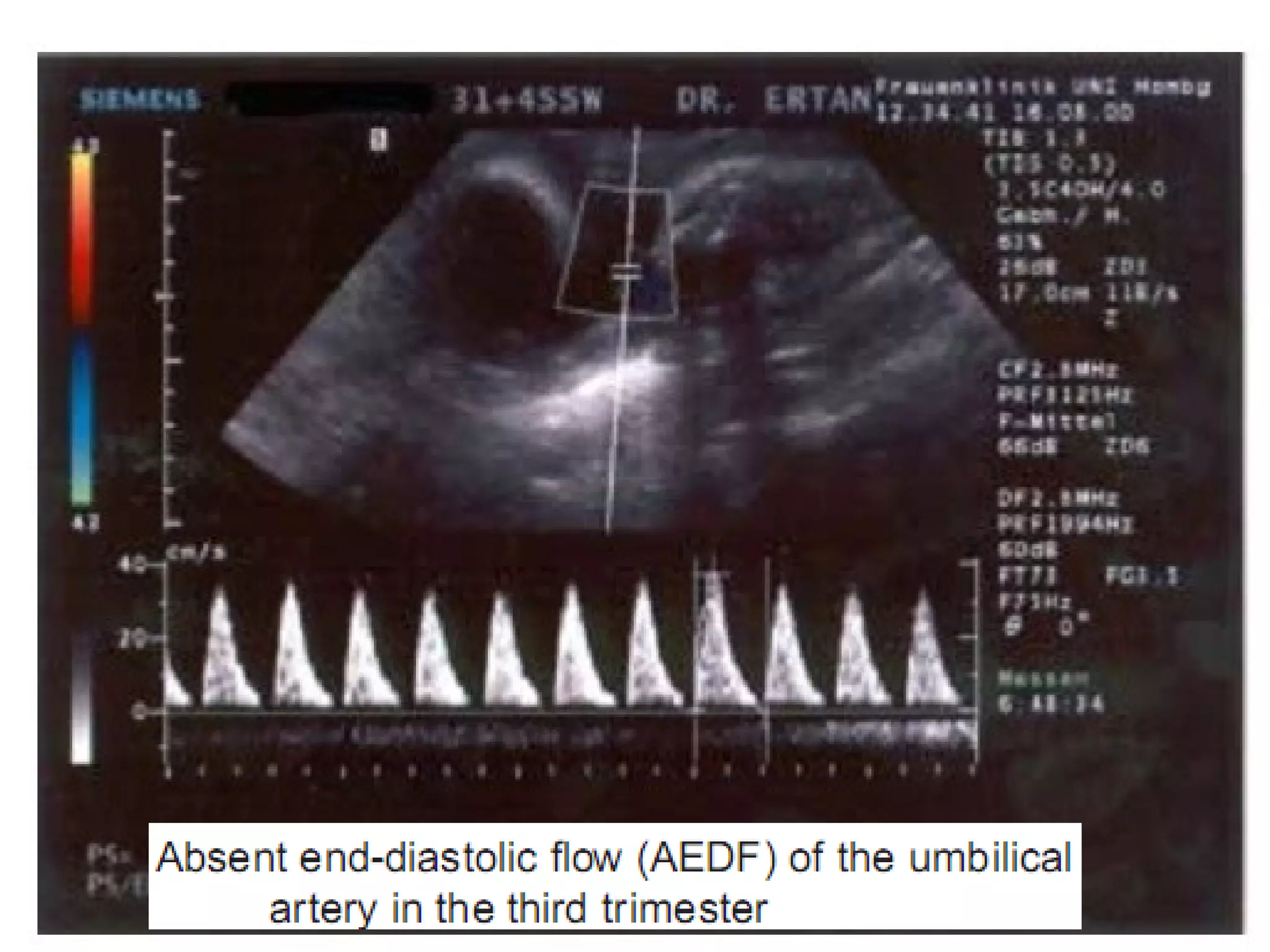
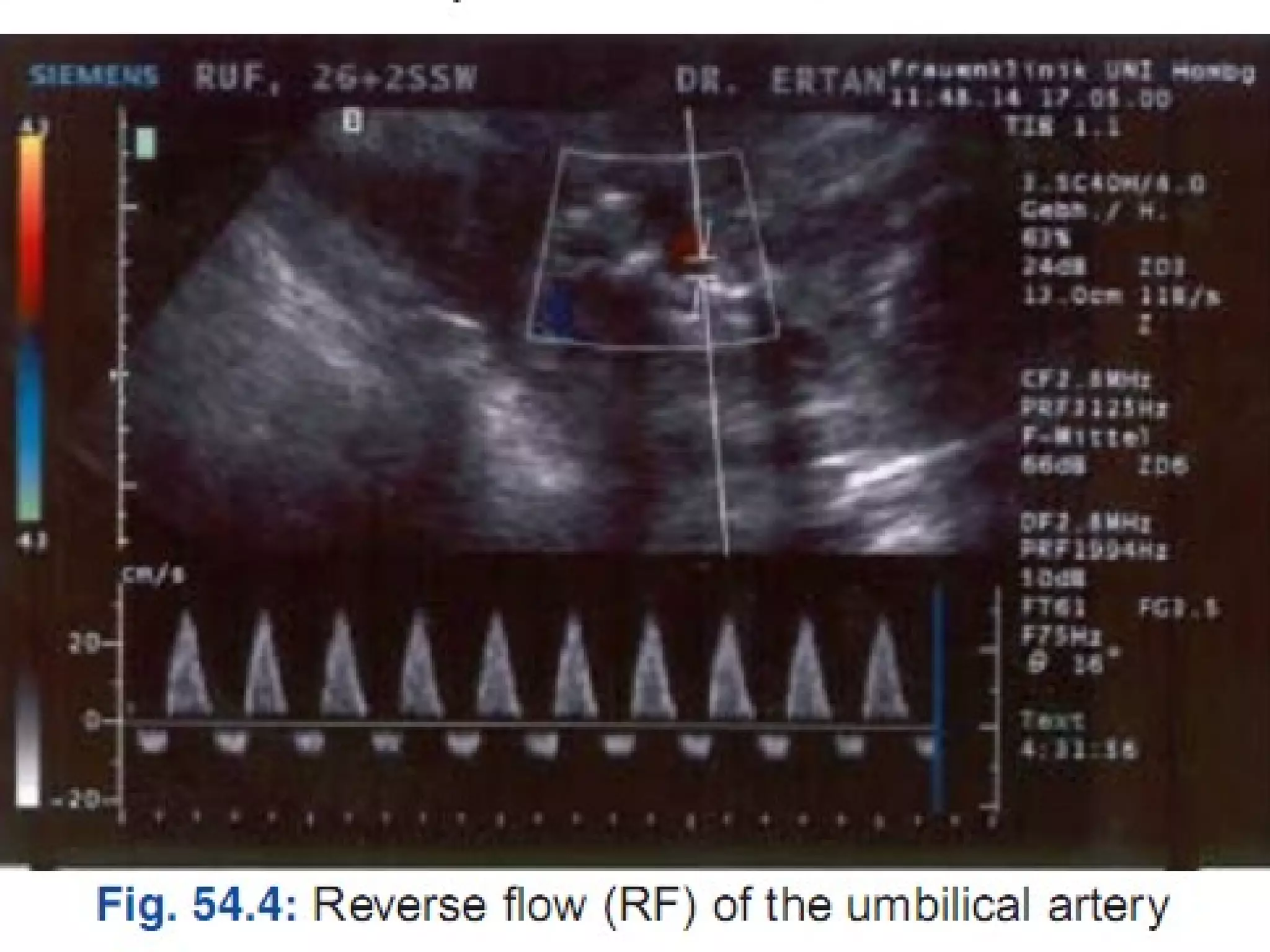
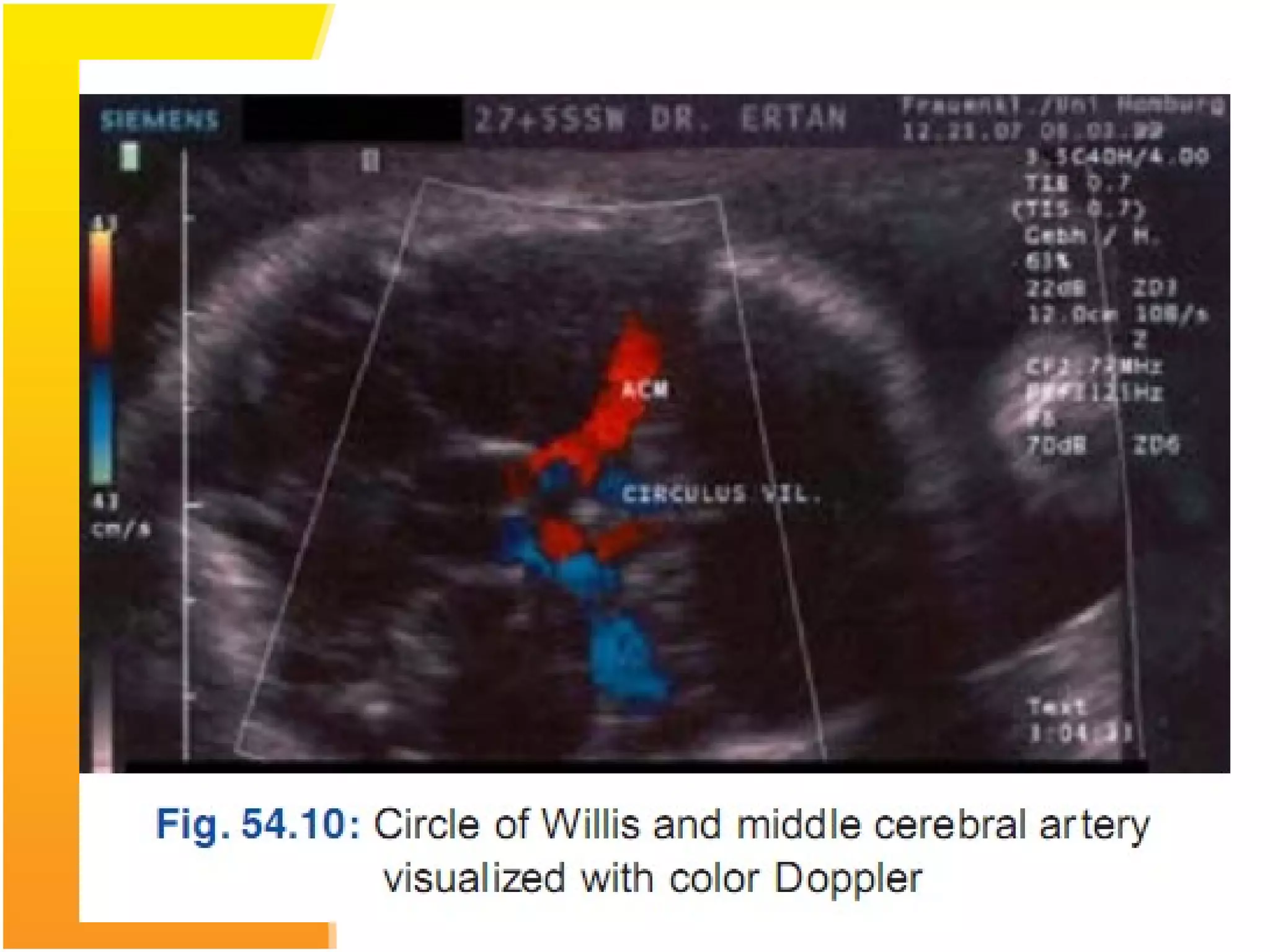
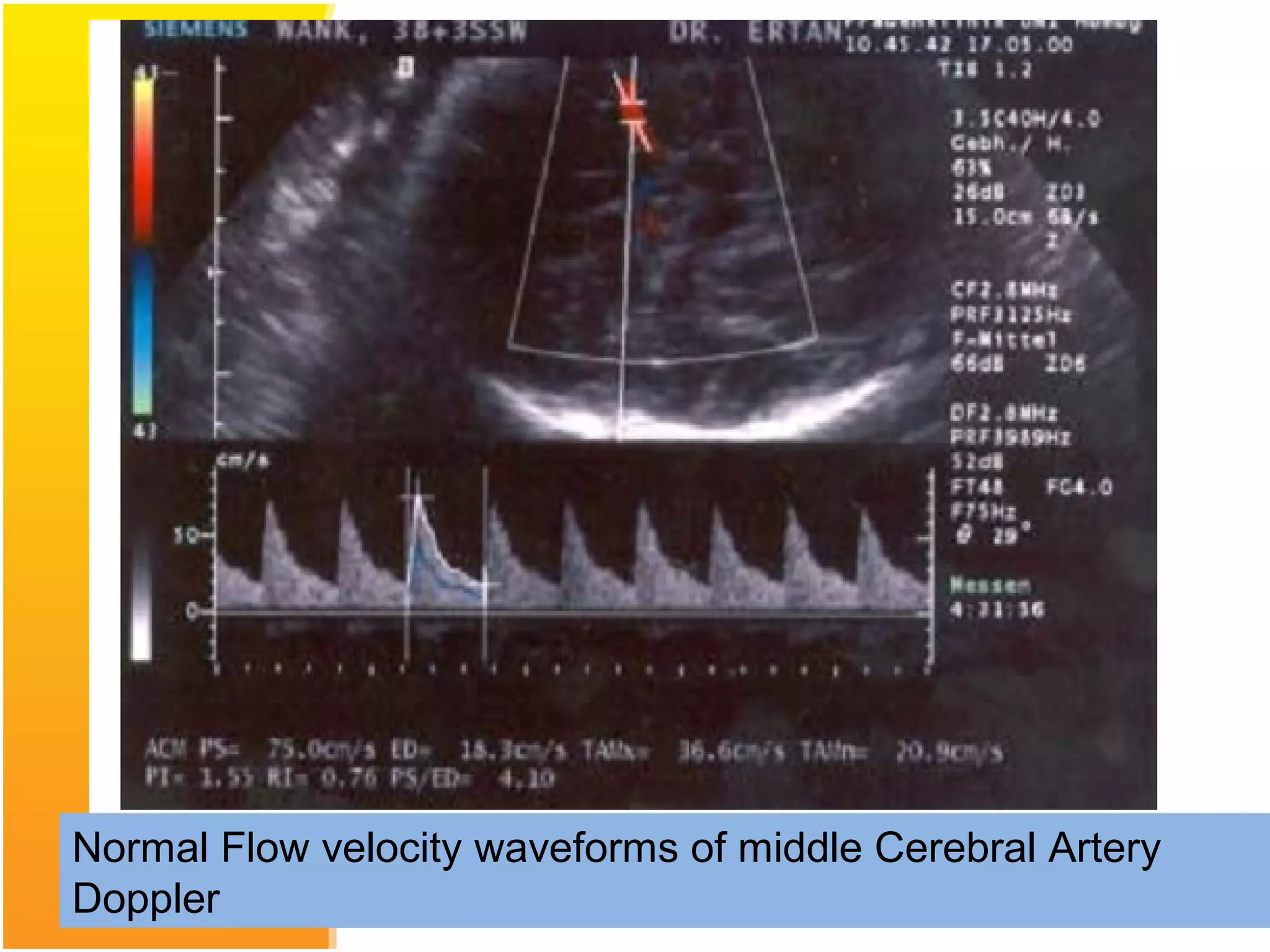
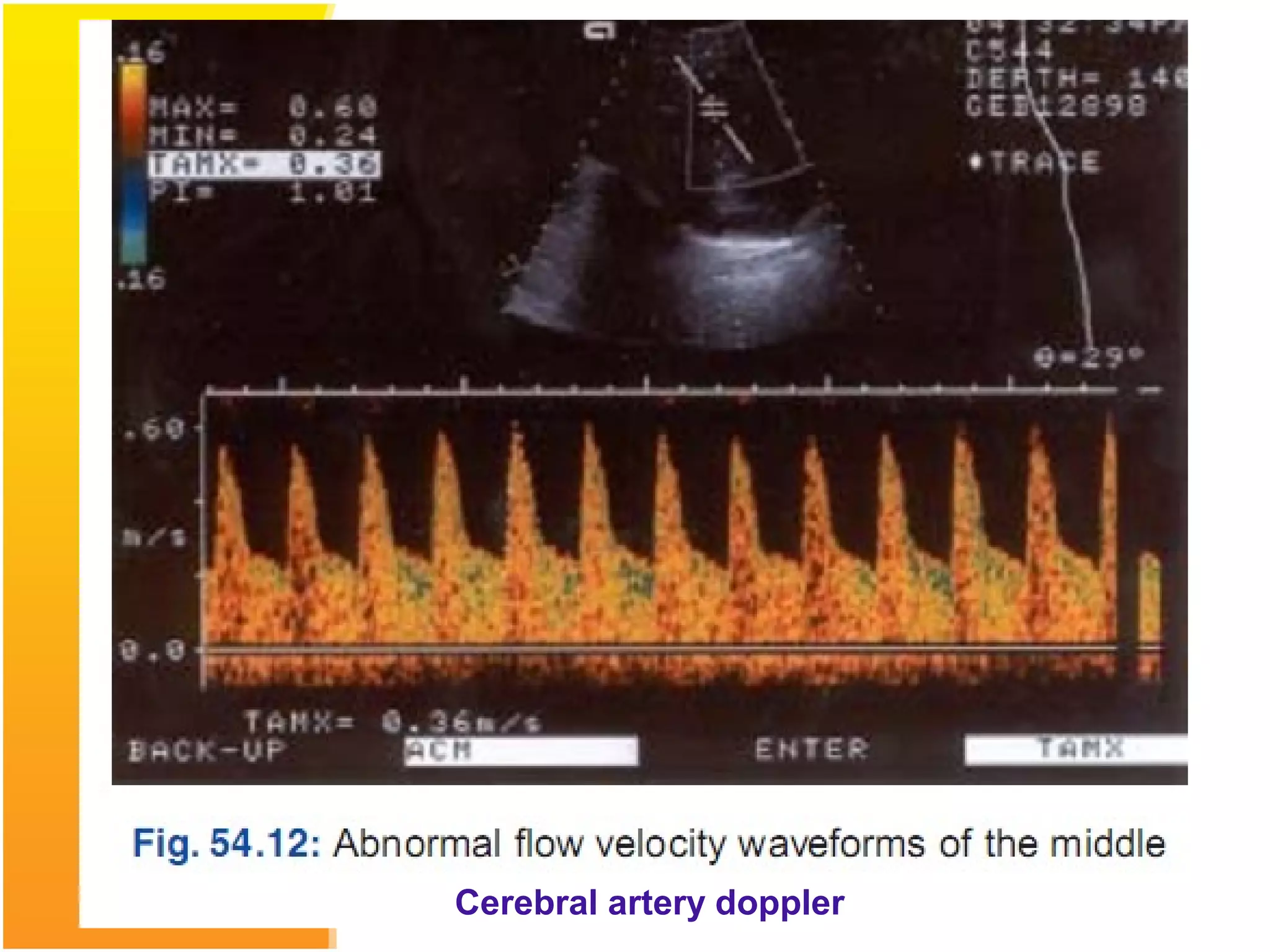
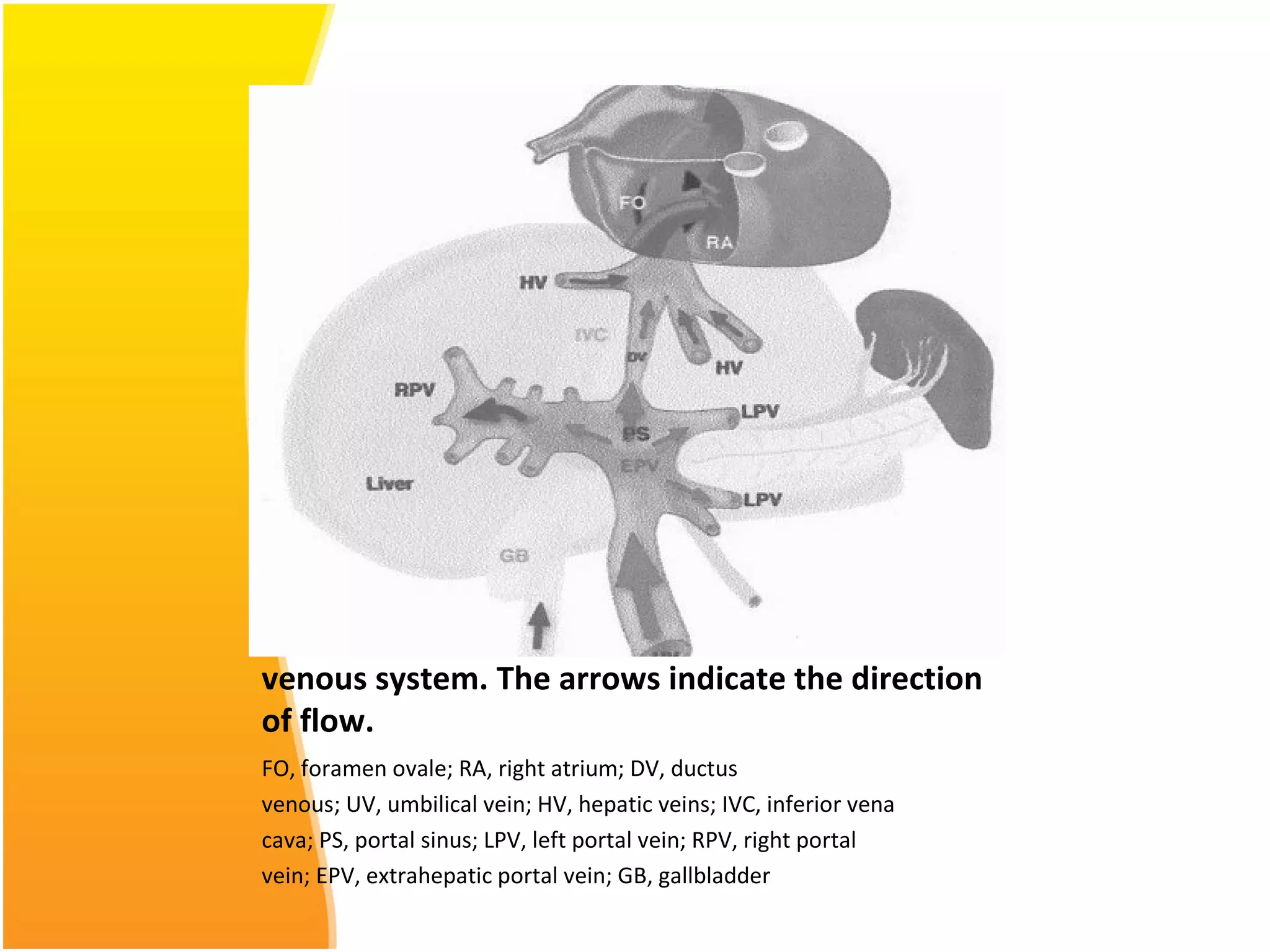
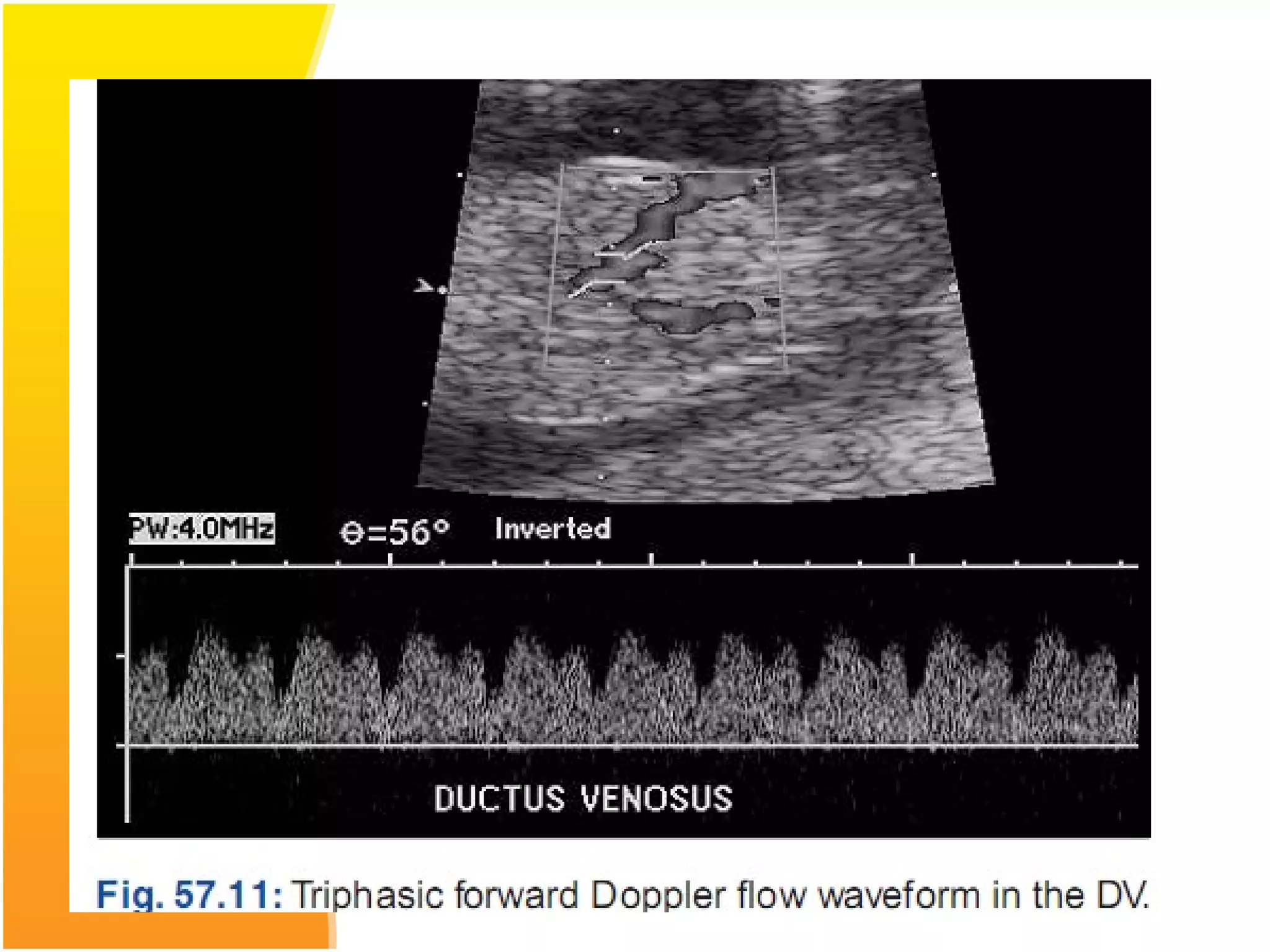
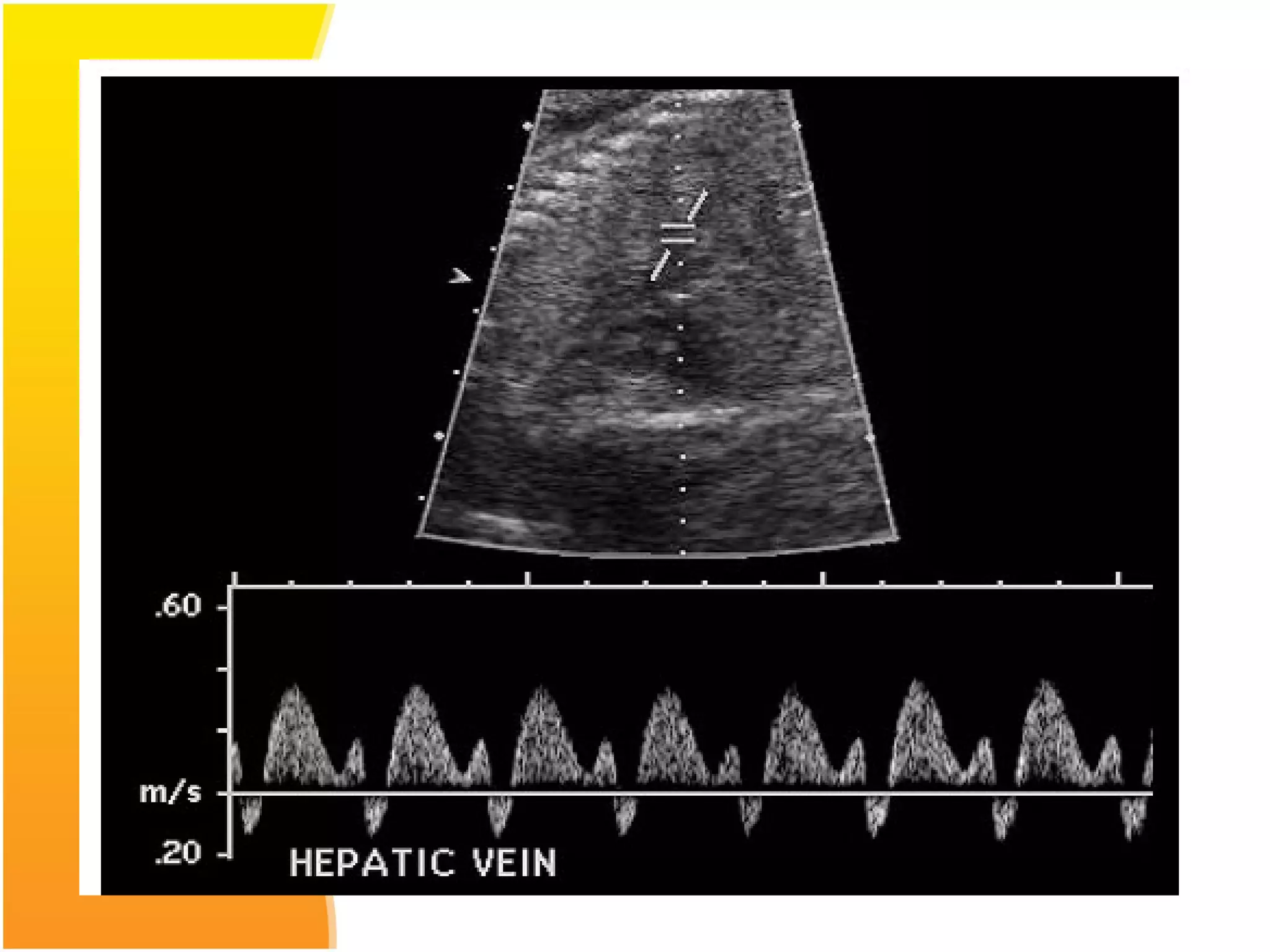
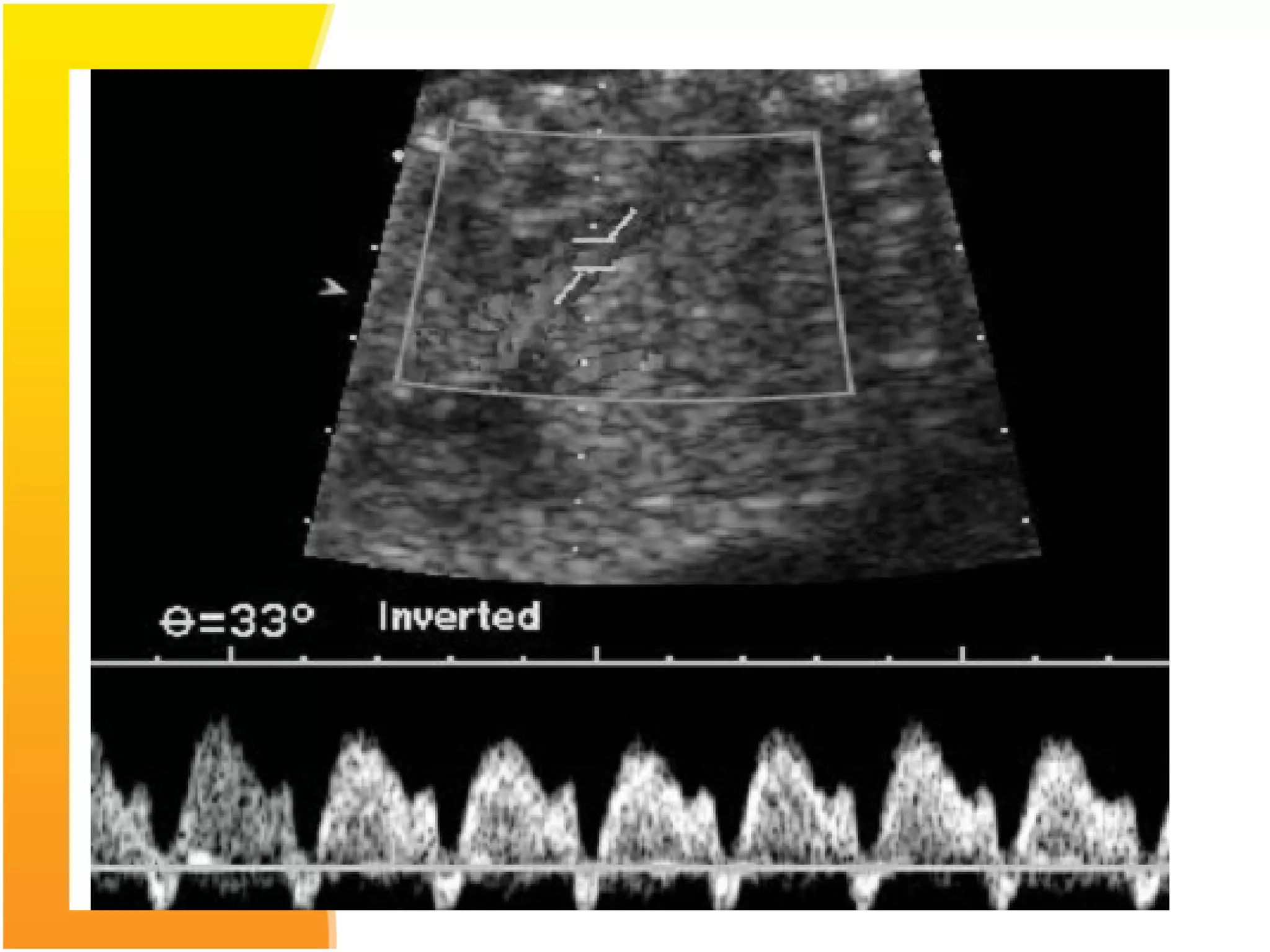
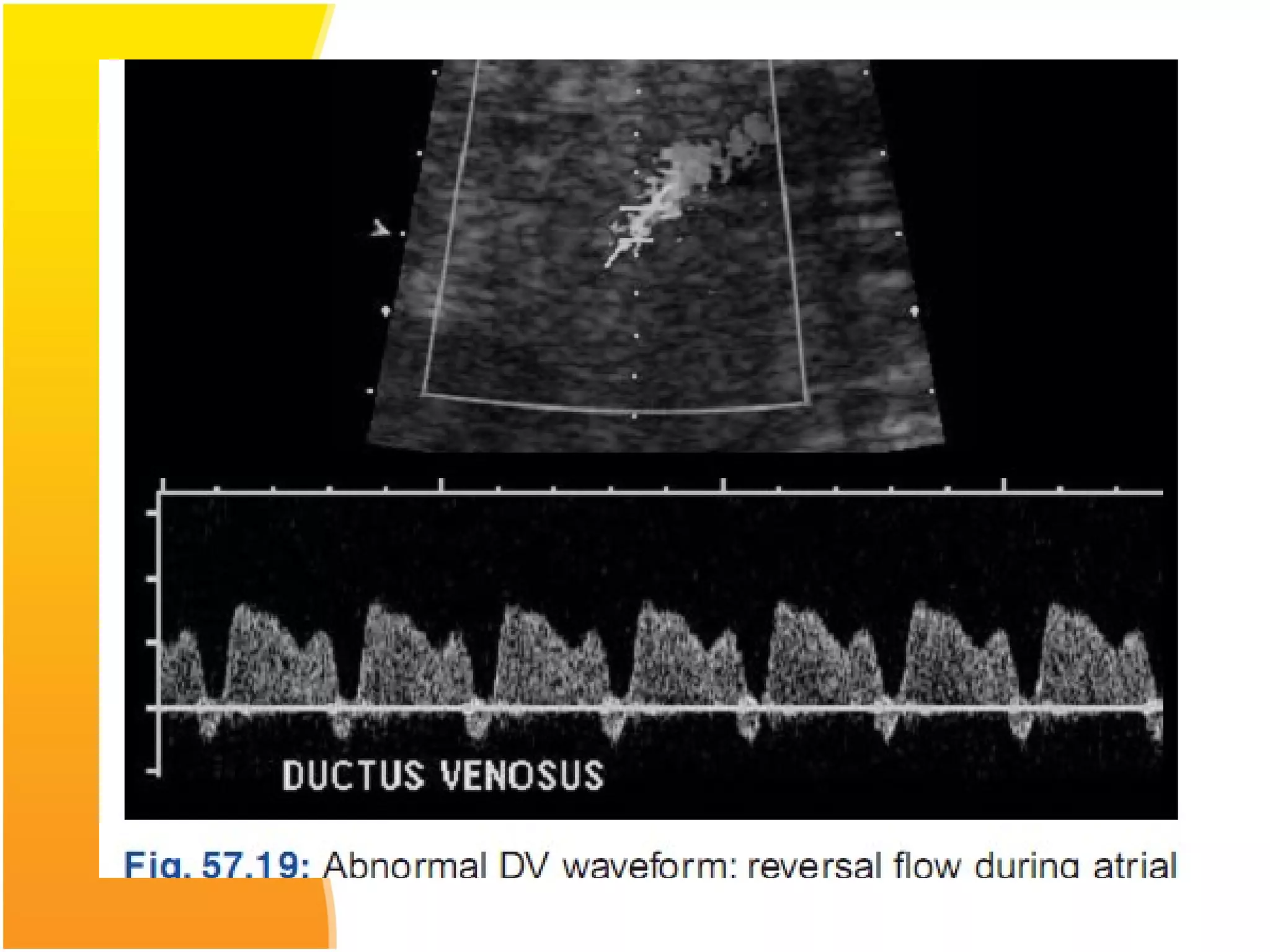
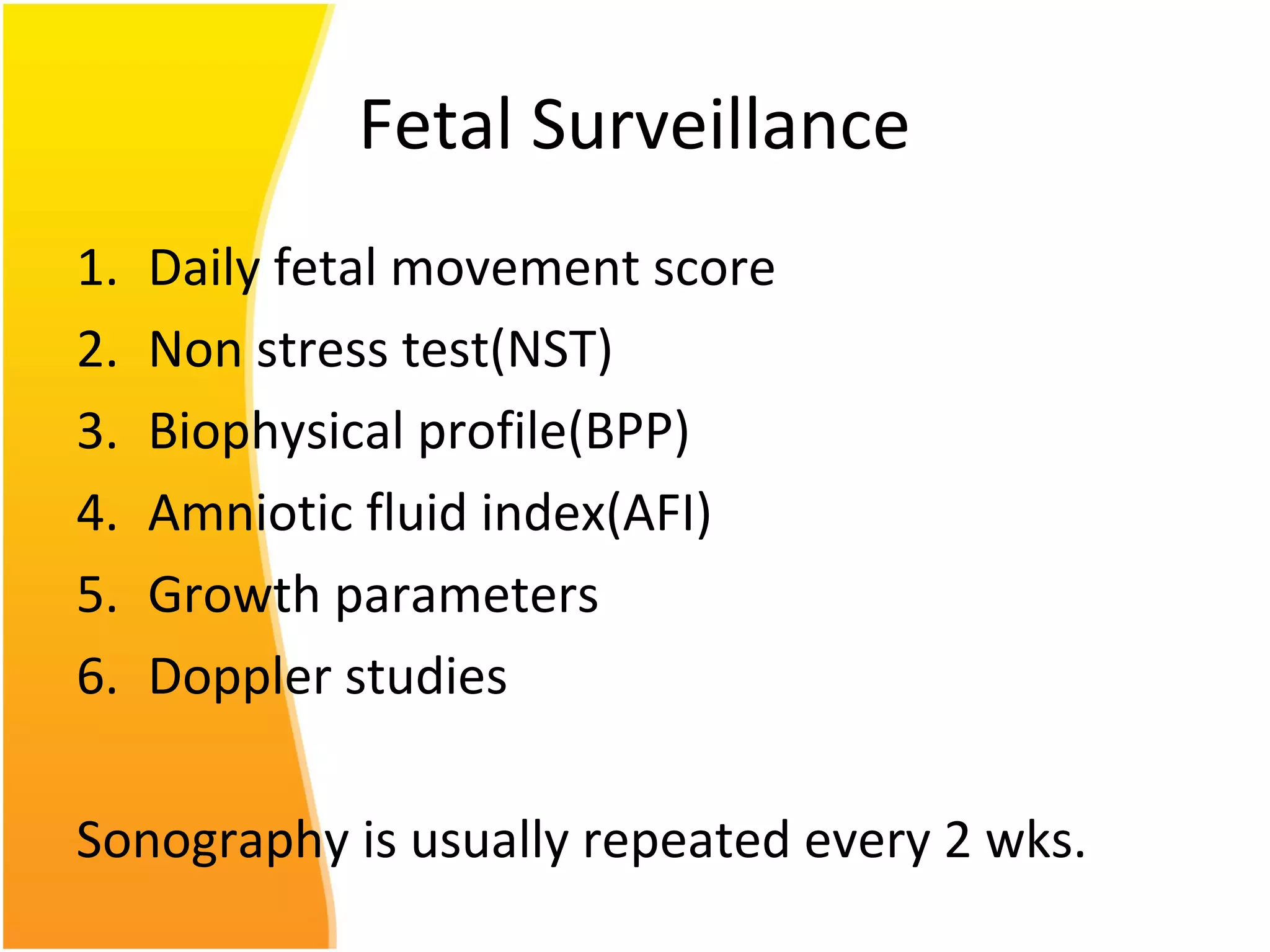
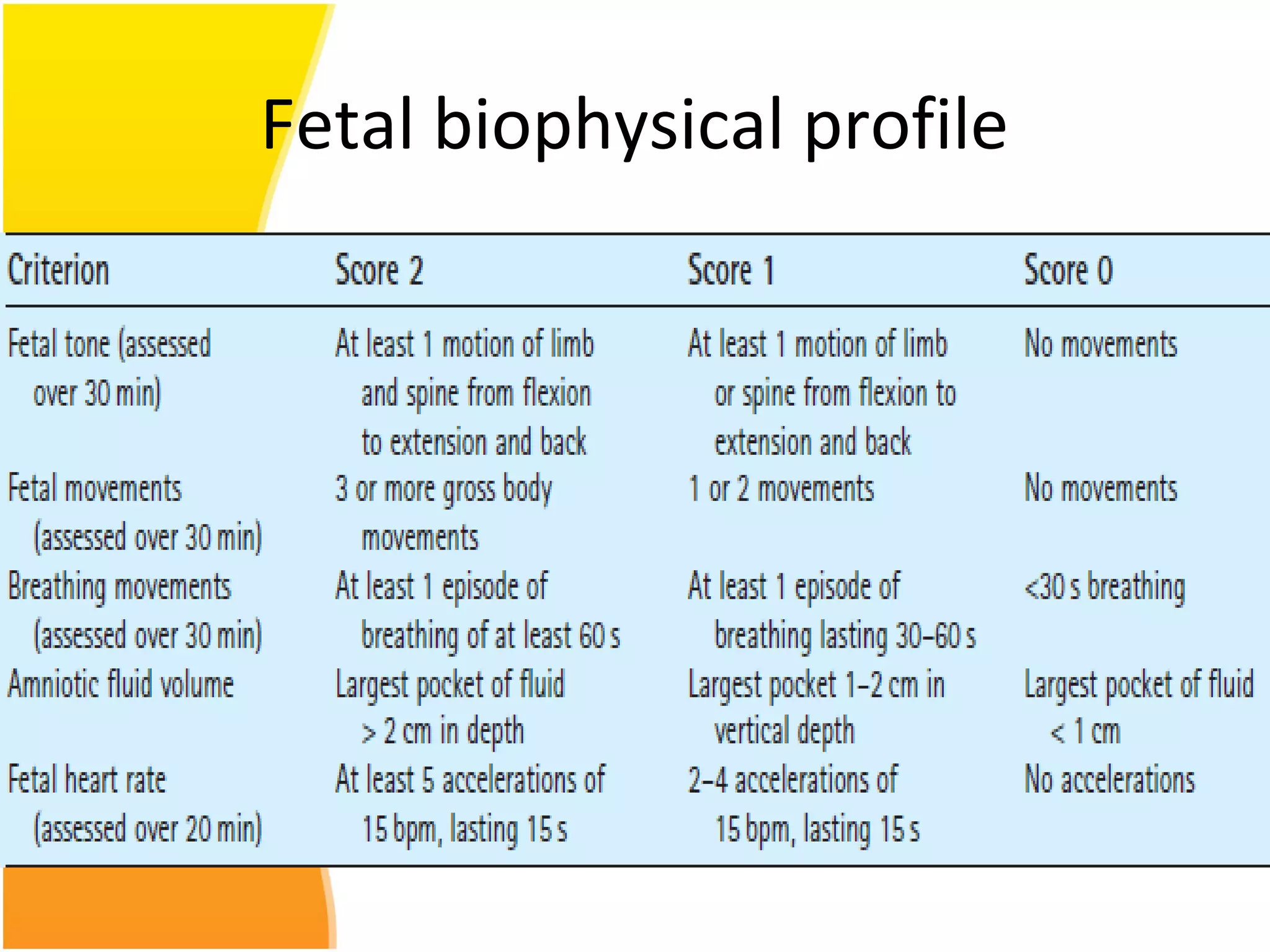
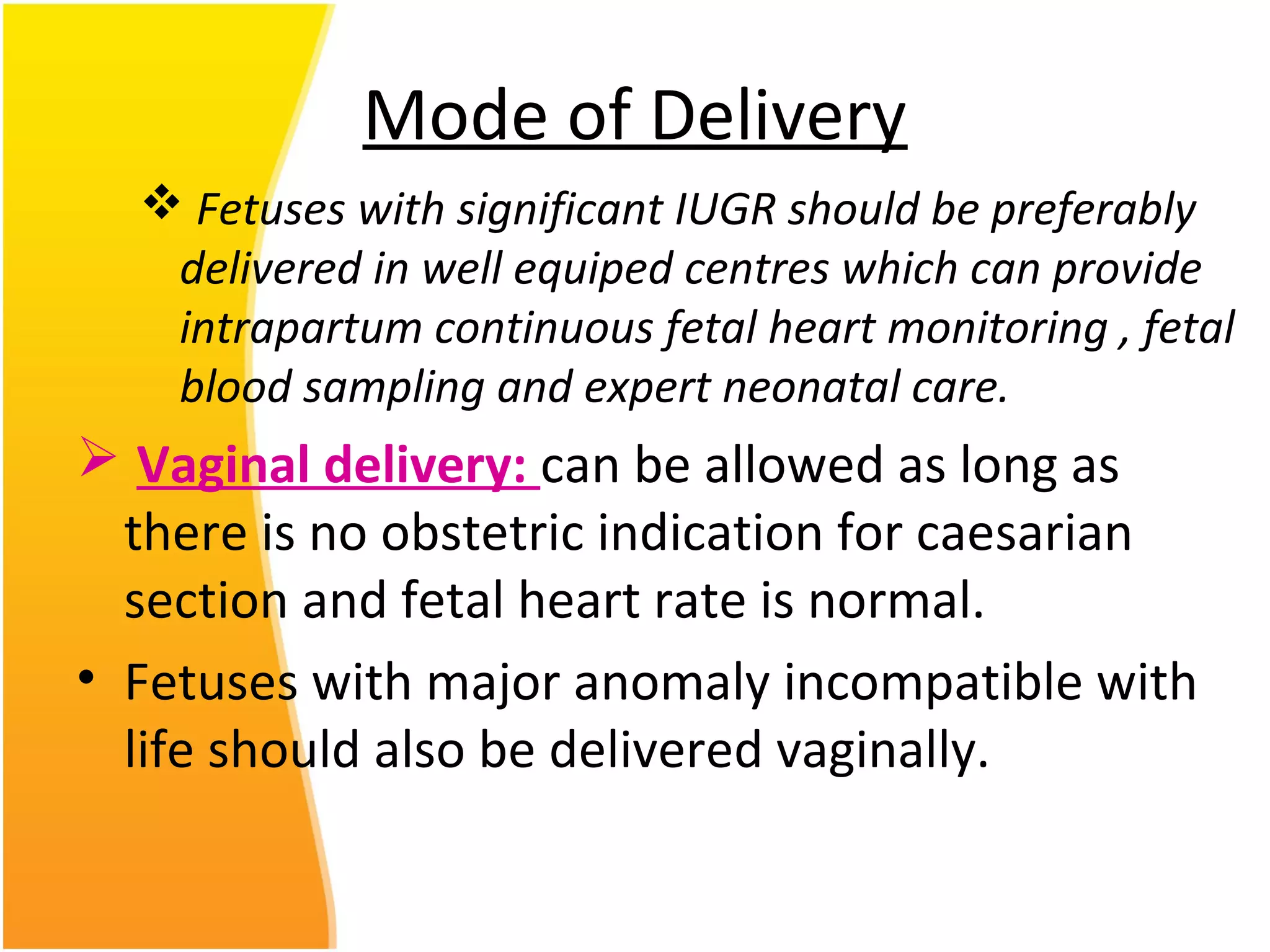
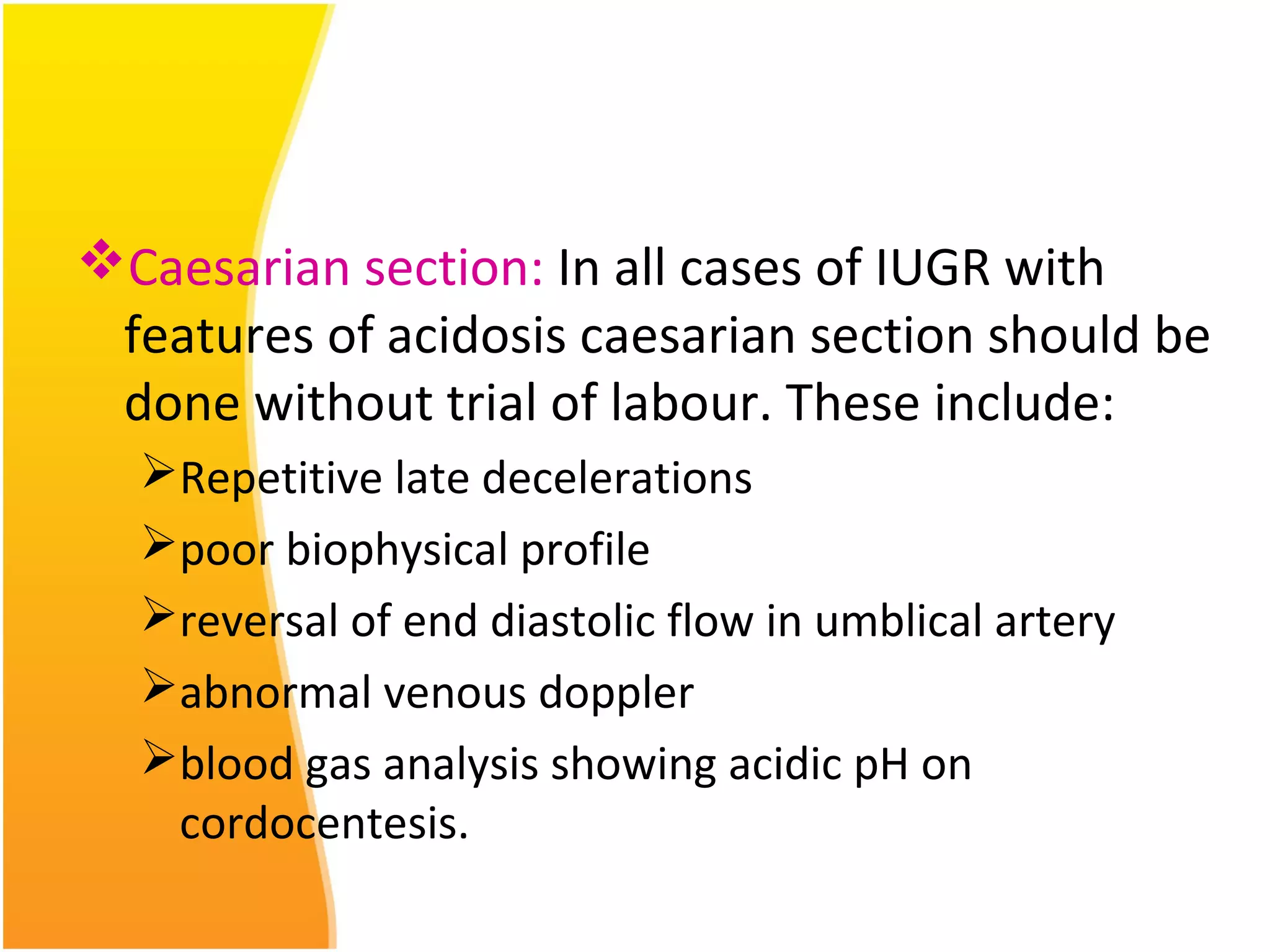
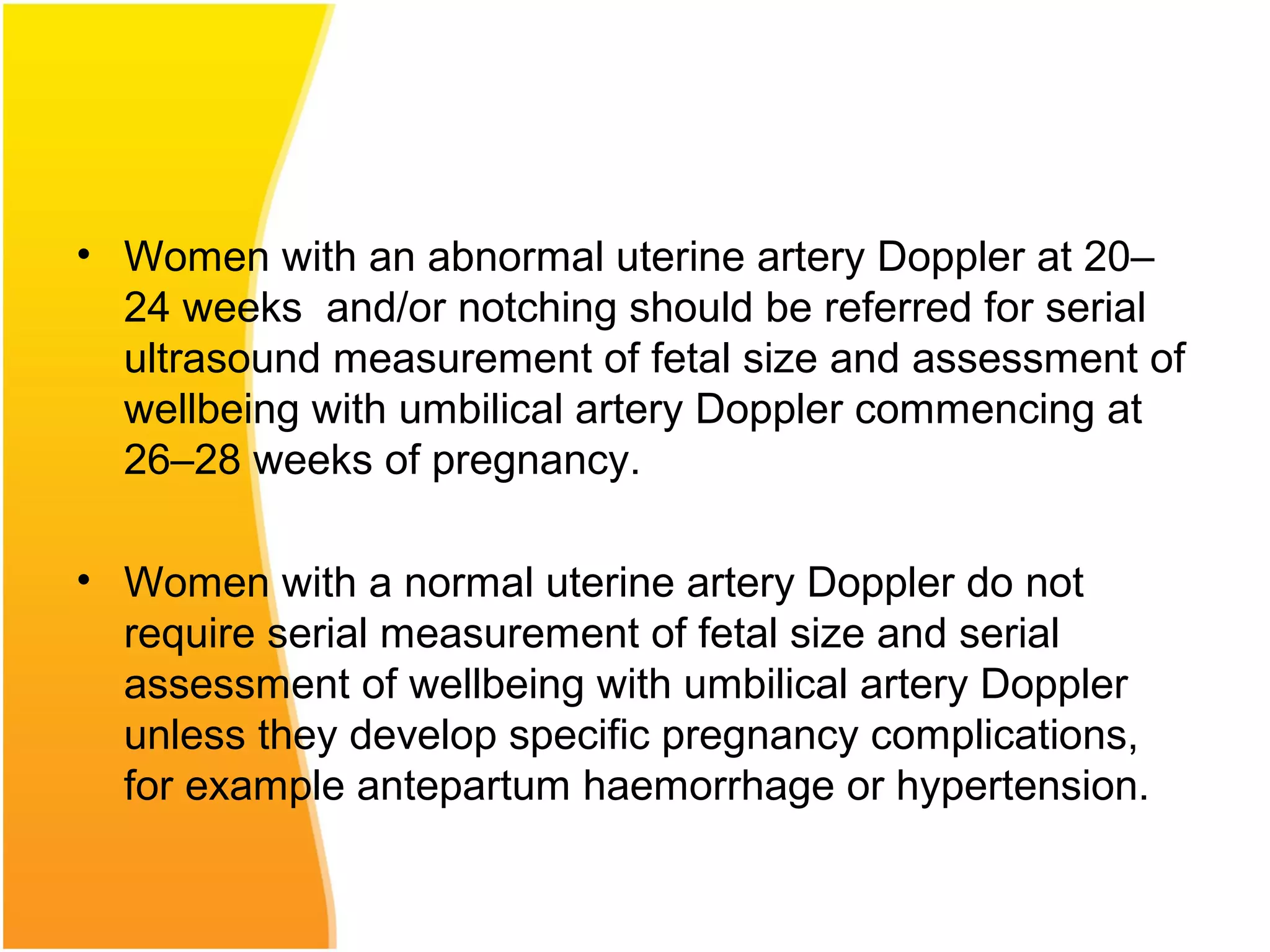
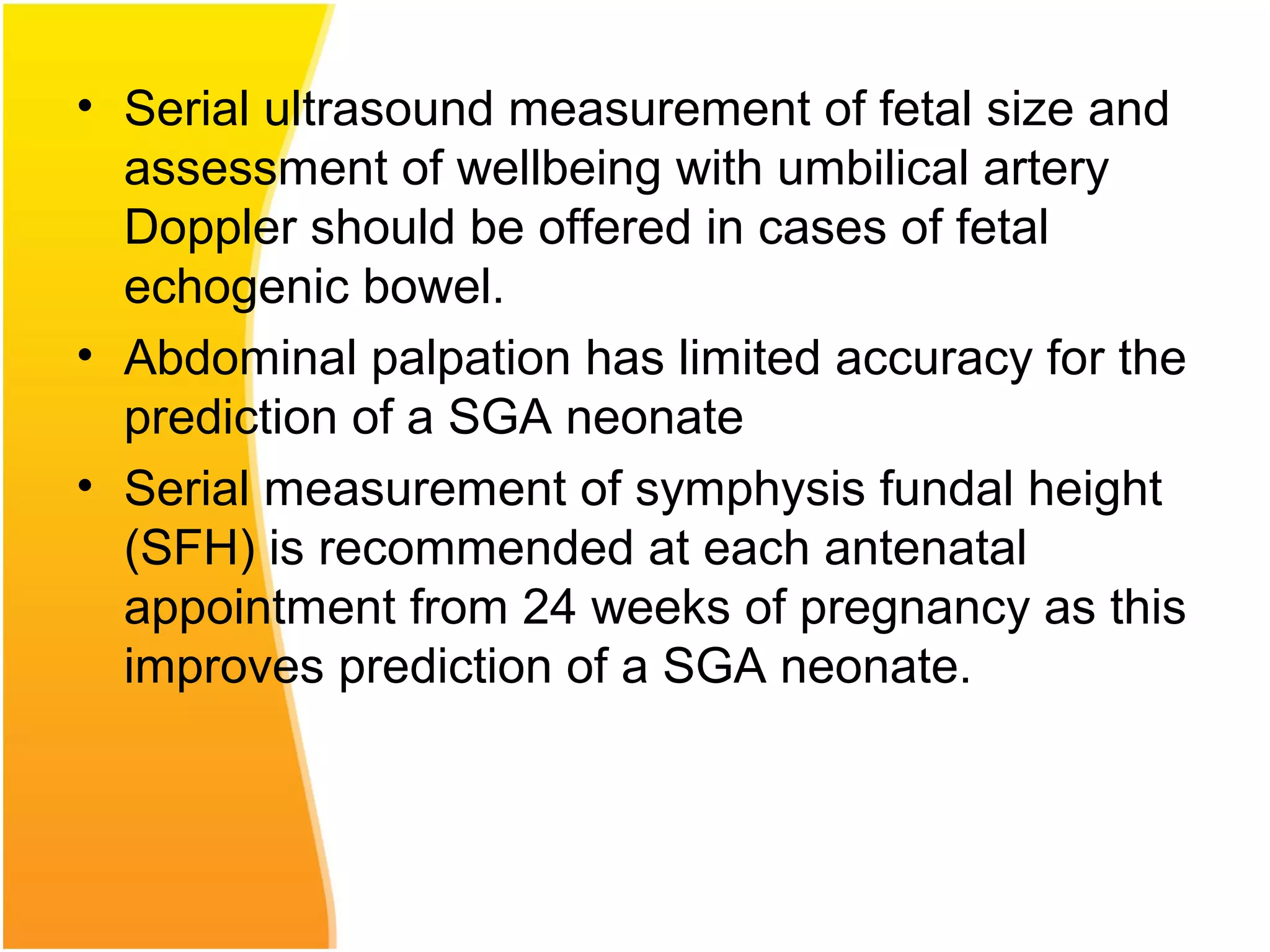
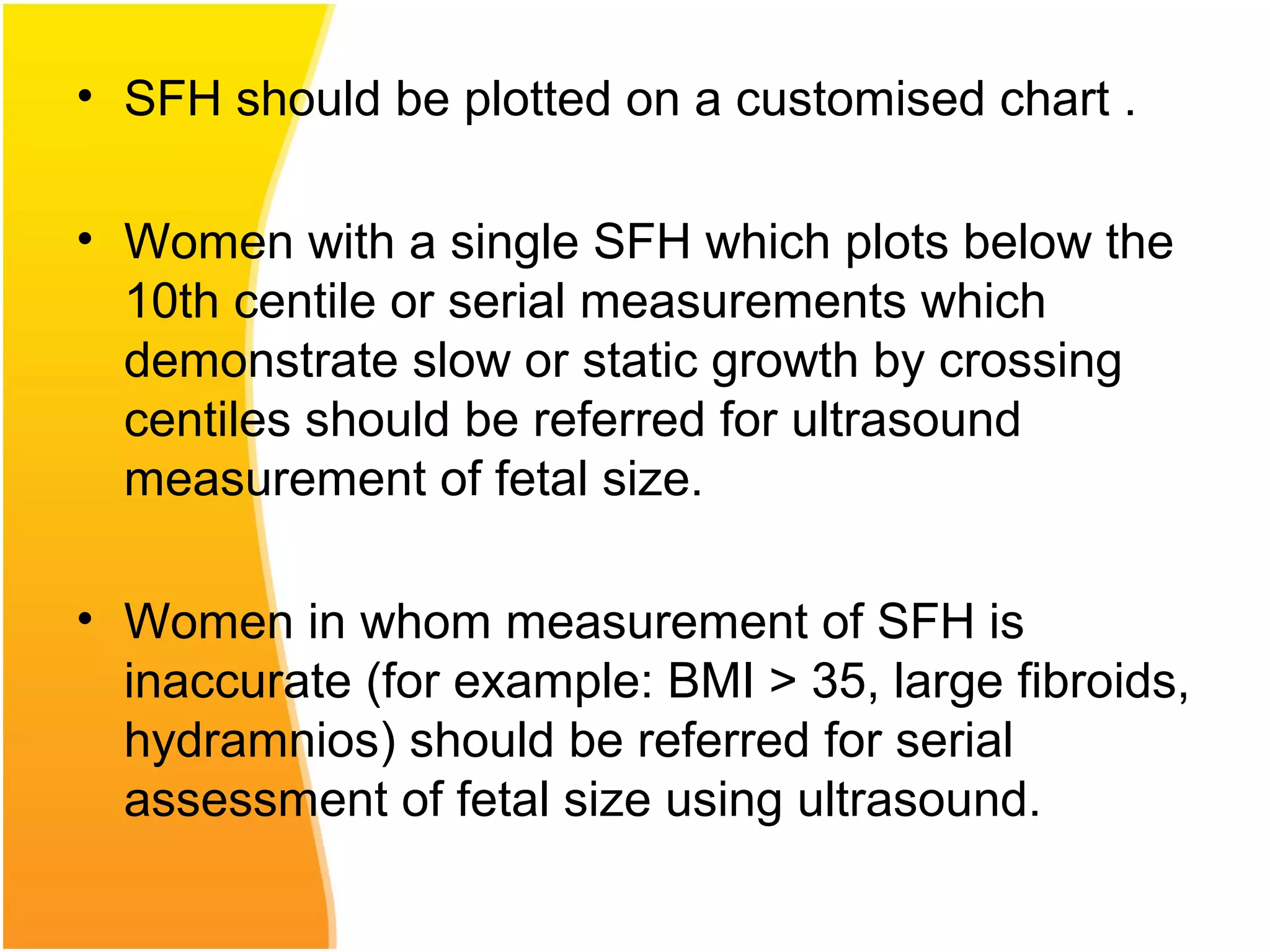
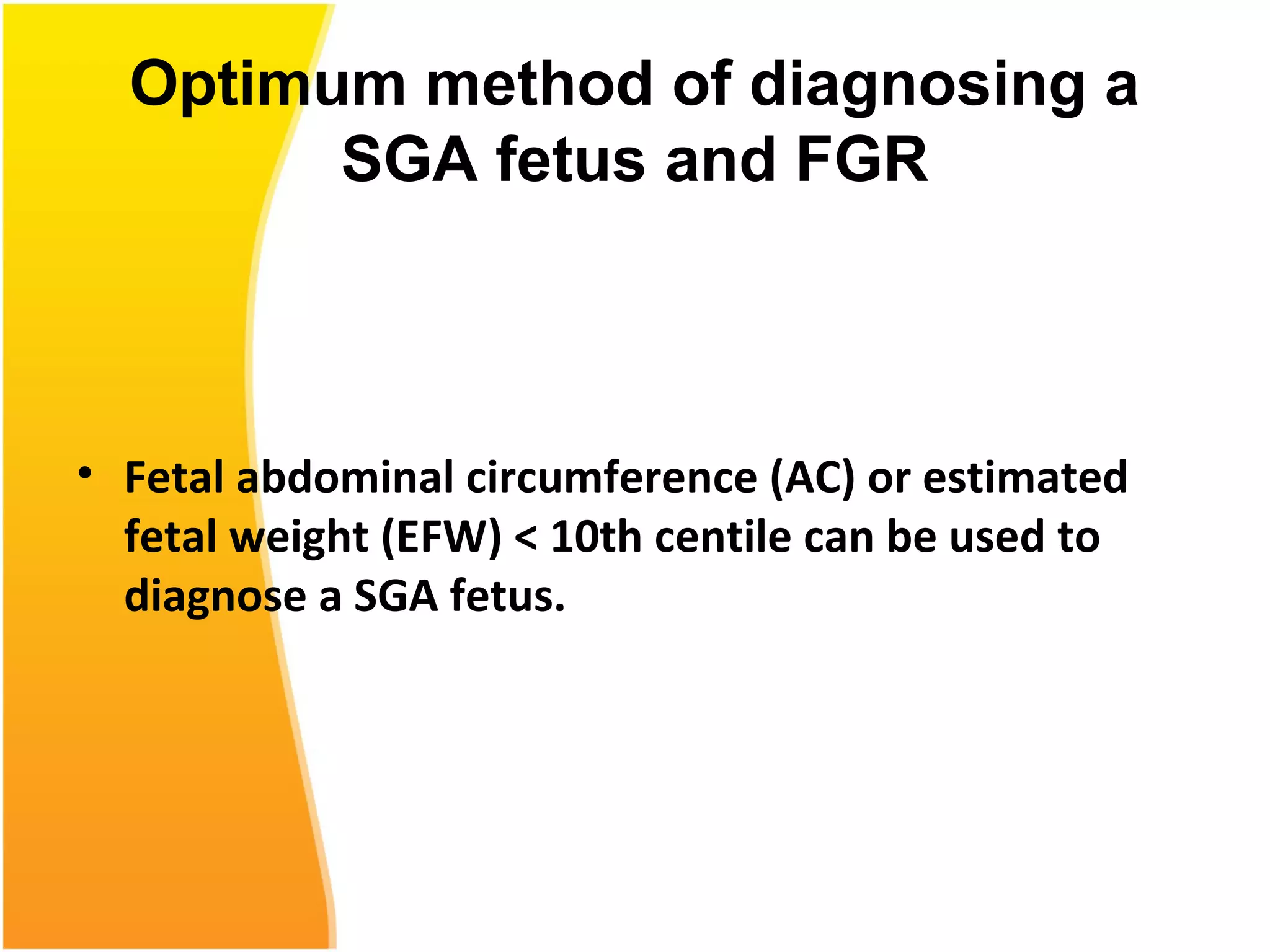
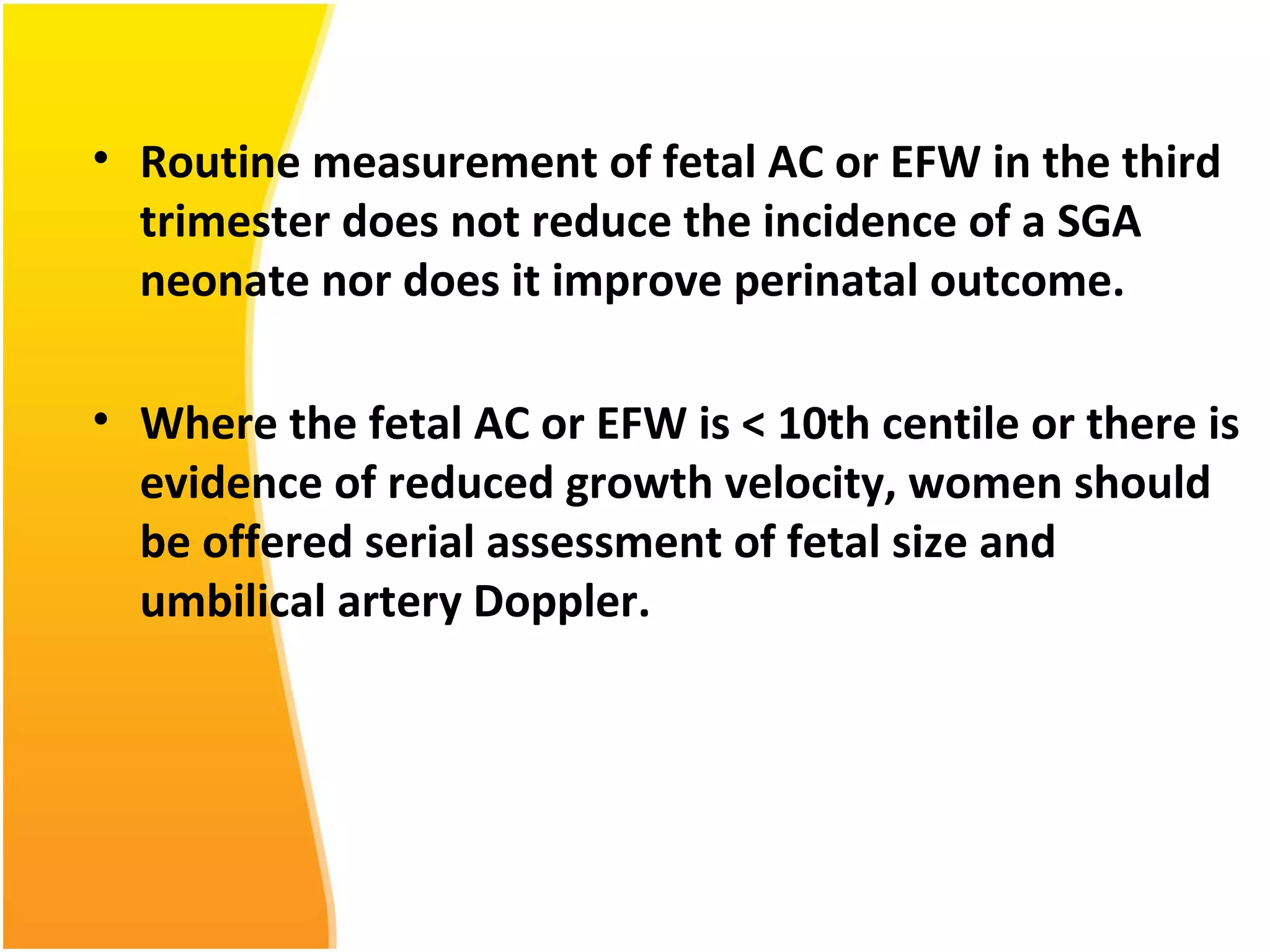
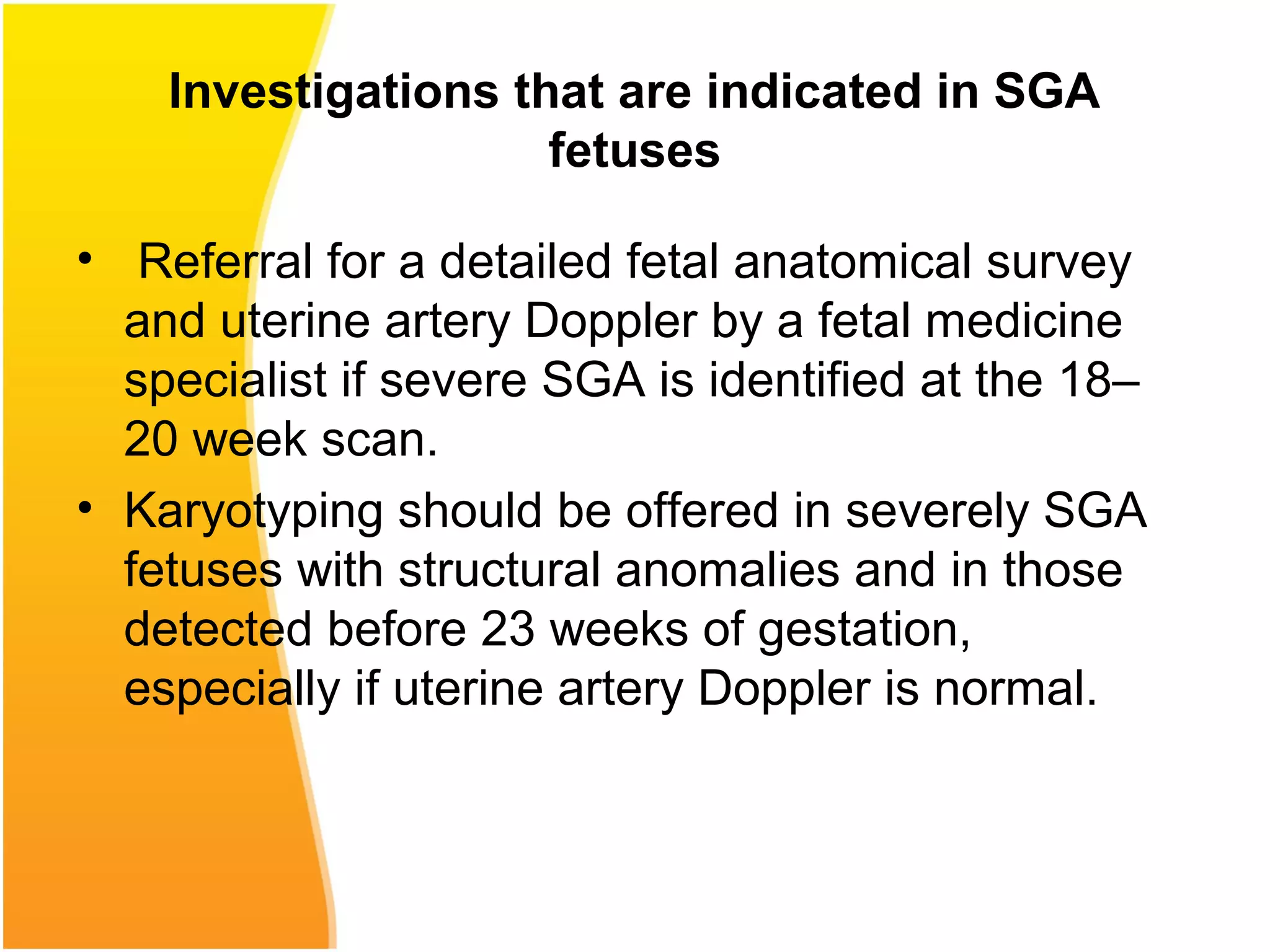
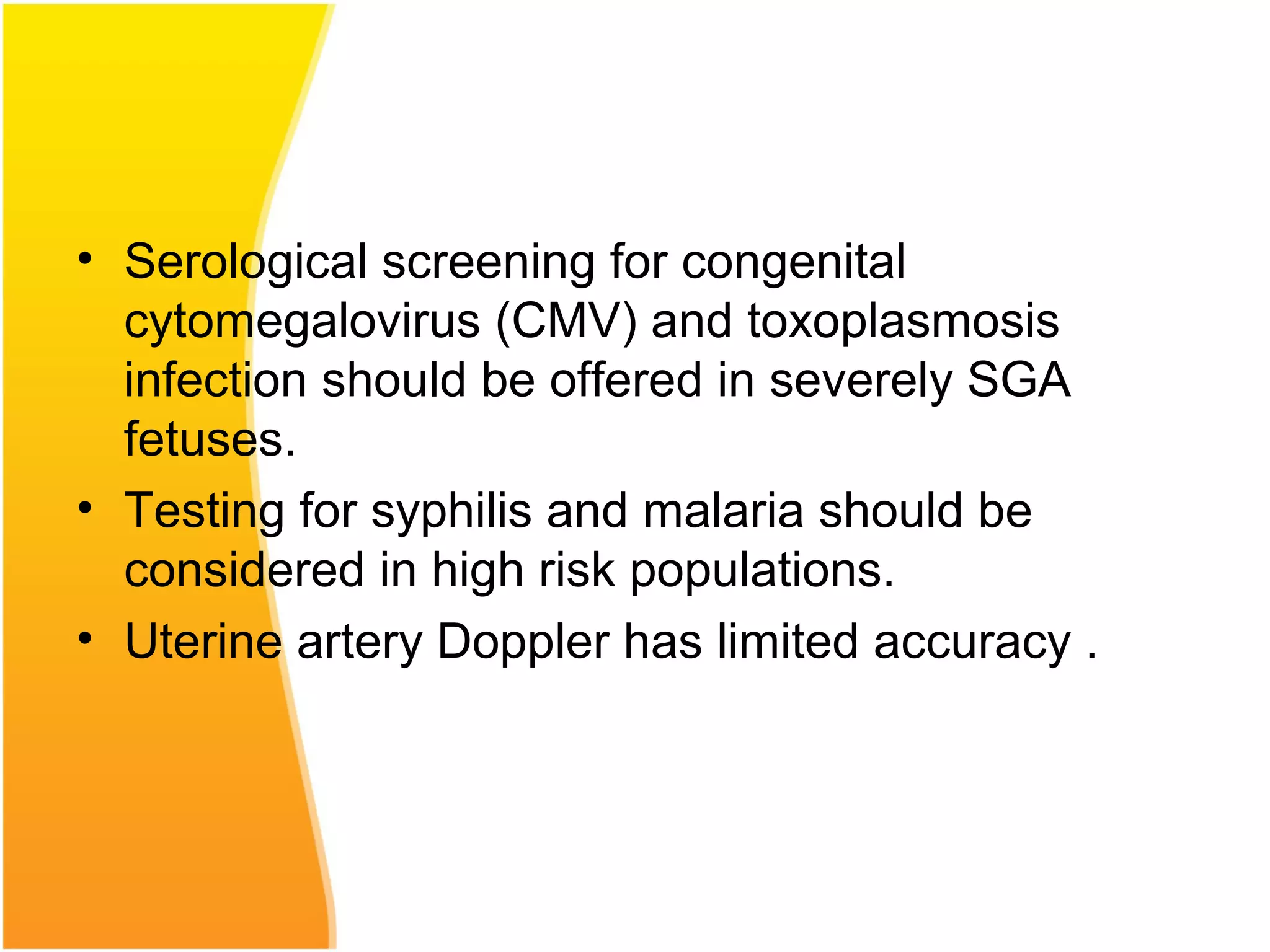
Intrauterine growth restriction (IUGR) refers to fetuses that are small for their gestational age. It can be symmetric/intrinsic or asymmetric based on whether all growth parameters are proportionally small or whether the head is spared. Symmetric IUGR is caused by early placental insufficiency affecting cell number, while asymmetric IUGR occurs later from reduced nutrient/oxygen supply, sparing the brain. Diagnosis involves serial fundal height measurements, ultrasound to assess growth parameters and placental grading, and monitoring for complications like stillbirth.