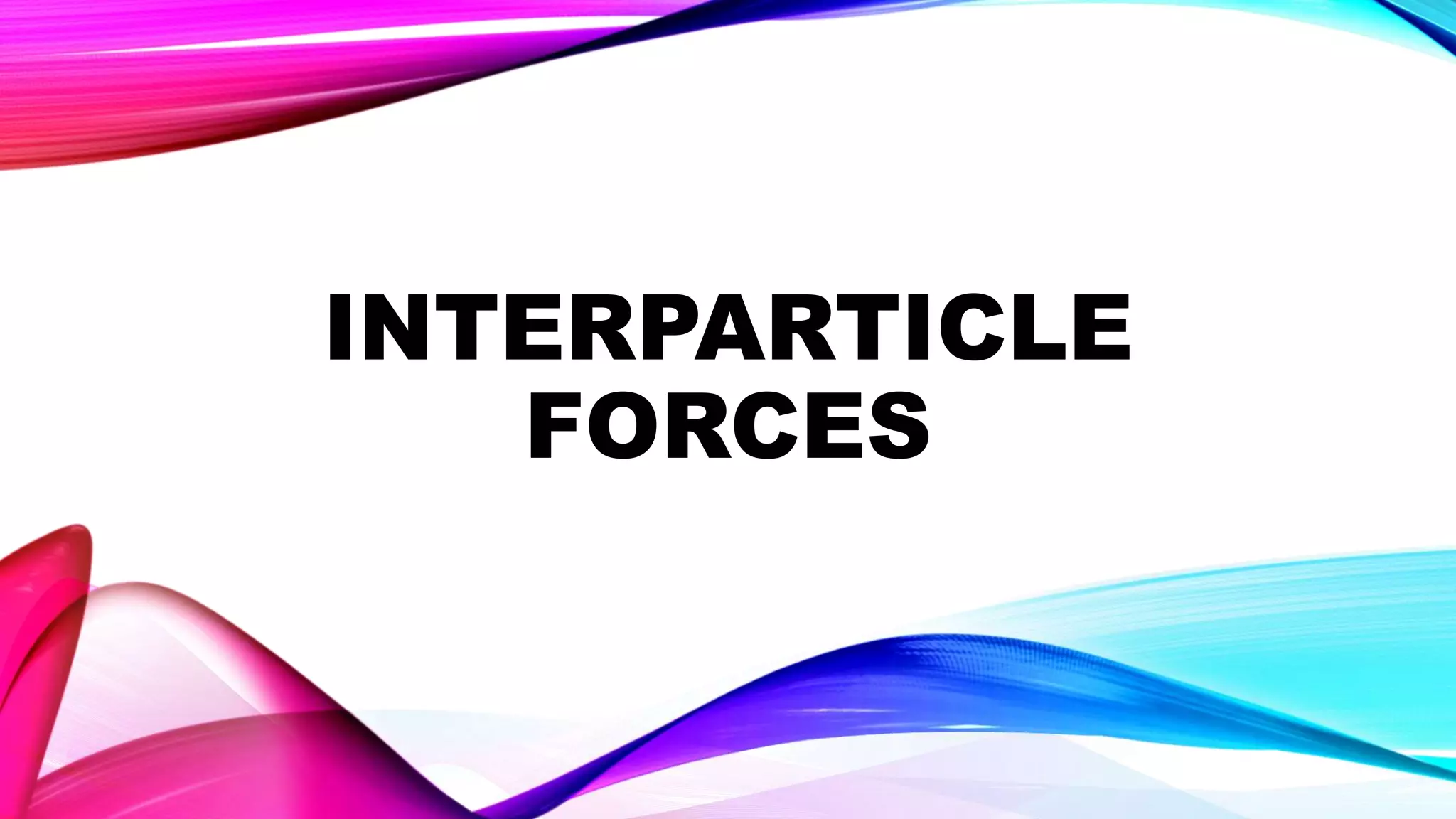
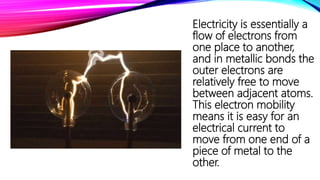
Solids have particles that are closely packed and held together by strong interparticle forces, giving solids their rigid structure with limited mobility. This makes solids incompressible with high melting points and heat of fusion. Metals conduct electricity and heat well due to movable electrons between atoms. Non-metals like graphite can also conduct due to unique bonding structures. Solids can be molded when malleable and drawn into wires when ductile. Solubility depends on bond strength, with molecular solids dissolving through weak intermolecular forces. Density relates to mass within a volume and is higher when particles are packed closely together.