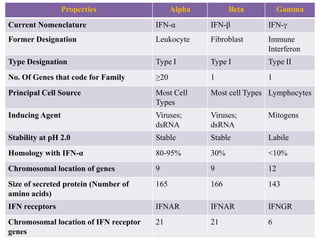
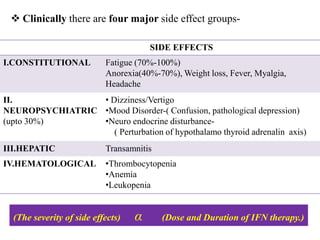
Interferons (IFNs) are cytokines that were first recognized for their ability to interfere with viral infections. There are three main types of IFNs - alpha, beta, and gamma - which are classified based on their antigenicity and receptor binding. IFNs have diverse antiviral, immune enhancing, and antiproliferative properties. They work by binding to IFN receptors on cells and inducing the expression of interferon stimulated genes, which leads to an antiviral state. IFNs have many therapeutic applications for treating viral infections and cancers, though they can also cause side effects like fatigue, mood changes, and liver/blood abnormalities. Viruses have evolved various mechanisms to counteract the effects of IFNs.