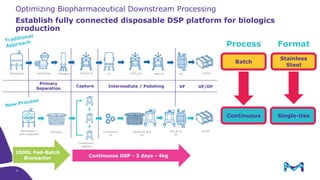
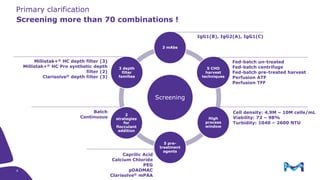
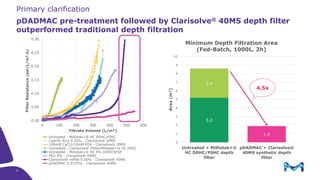
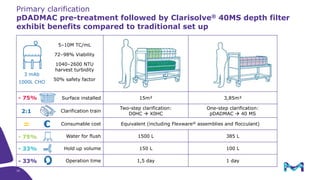
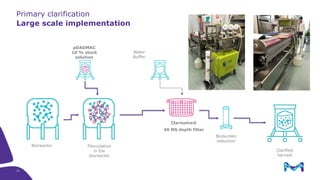
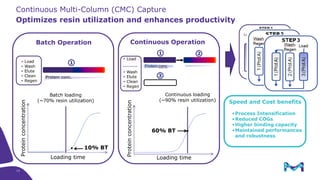
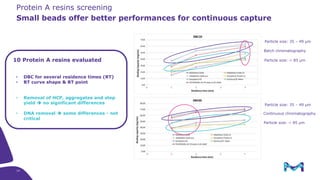
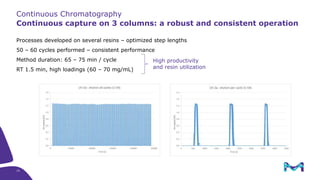
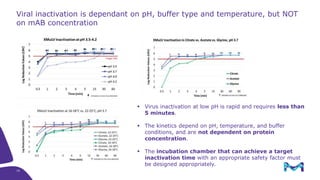
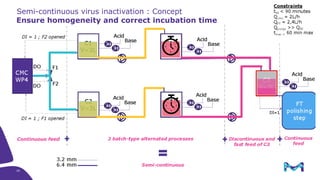
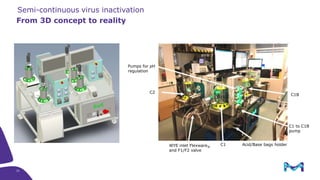
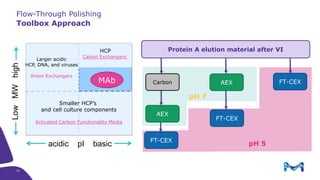
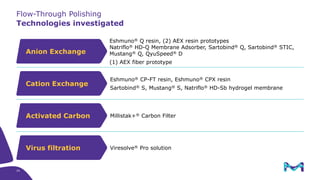
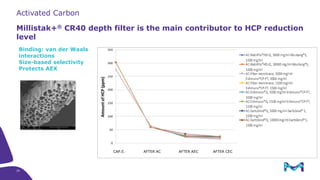
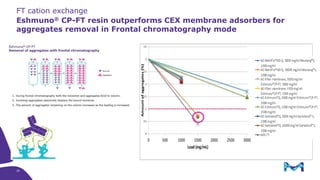
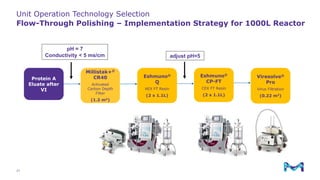
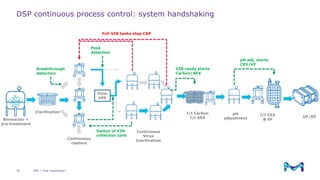
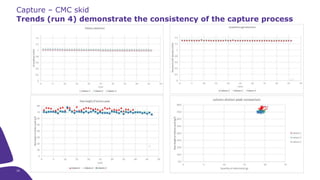
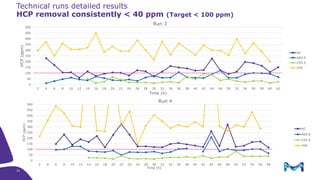
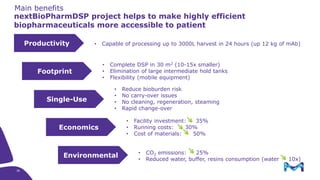
The document outlines the implementation of a fully single-use, integrated monoclonal antibody biosimilars purification platform by Merck KGaA as part of a four-year project funded by the EU Horizon 2020 program. The initiative aims to create a more efficient and environmentally friendly downstream process for manufacturing biopharmaceuticals, featuring innovative techniques like continuous capture and viral inactivation. The project showcases successful technical runs at a 1000L scale, leading to significant improvements in productivity, cost reduction, and bioburden risk management.