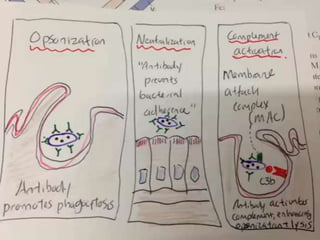
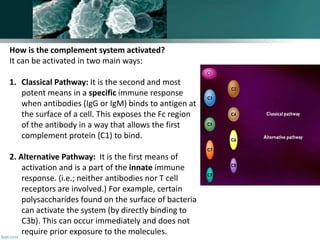
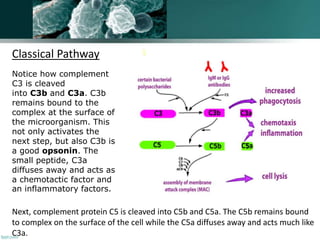
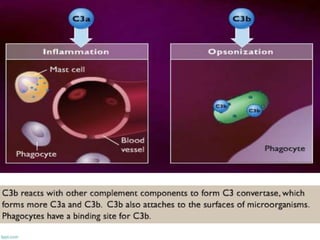
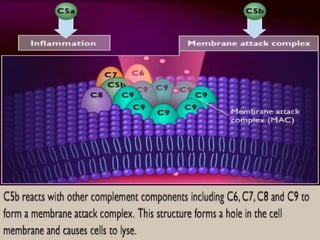
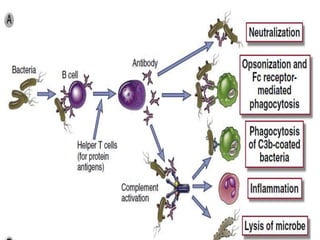
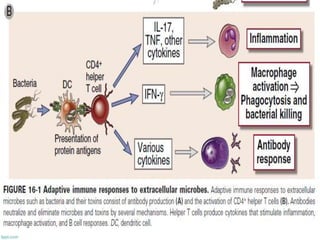
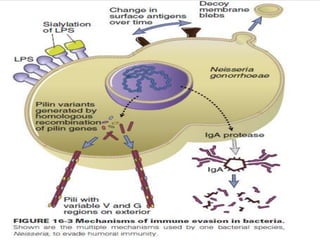
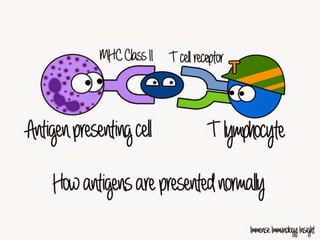
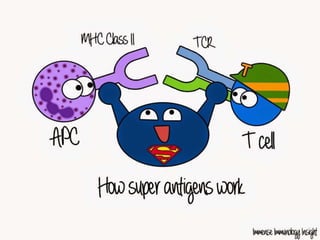
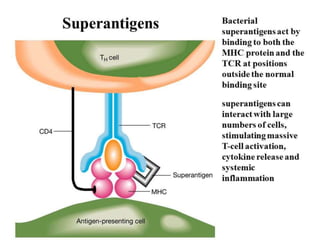
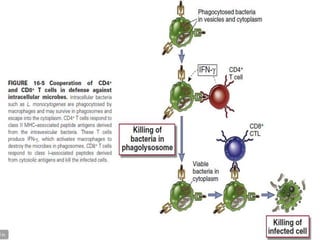
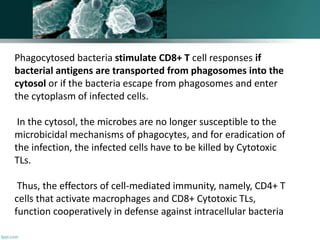
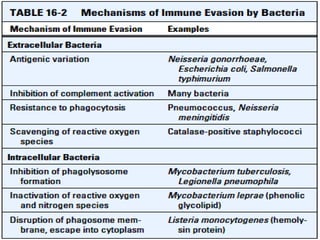
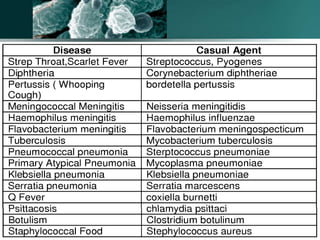
The document discusses the immune response to extracellular and intracellular bacteria, detailing mechanisms such as inflammation, toxin production, and the roles of complement systems and phagocytes. It explains how bacteria evade immune responses through strategies like antigen variation, capsule formation, and inhibiting immune activation. Additionally, it covers the consequences of immune responses, including septic shock and the chronic infections caused by intracellular bacteria due to their resistance to phagocyte elimination.